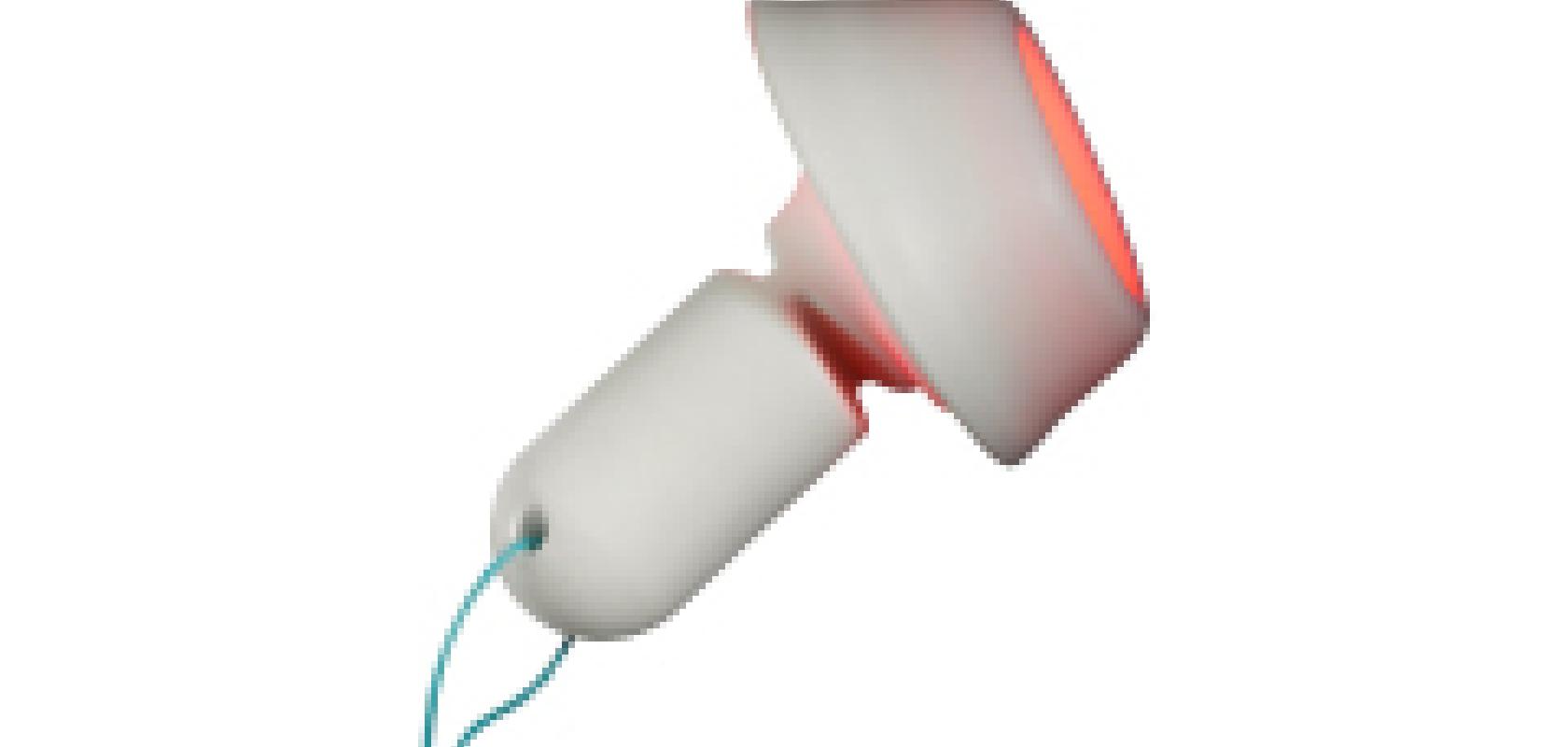
Global innovation specialist Sagentia has revealed details of its role in developing a new non-surgical tissue-preserving therapeutic procedure to more effectively remove HPV infection and treat precursors of cervical cancer. The light-activated drug treatment, named Cevira, is a drug-device combination procedure which has been accepted for use in a Phase II clinical trial by the US FDA. Trials will take place in the USA and in the EU. Cevira delivers a targeted light-activated treatment (also known as photodynamic therapy or PDT) intended to be used to destroy tissue infected by Human Papilloma Virus (HPV) and treat precancerous lesions on the cervix, without damaging healthy tissue.
The technique has been developed by Photocure, a Norwegian pharmaceutical company focused on dermatology and cancer, with Sagentia serving as global product development partner for the device. The self-powered, disposable device contains a red LED light source that, in combination with a medicinal product, initiates a photochemical reaction in exposed tissue. The fully integrated single-use device is easily administered by a trained gynaecologist or colposcopist and is then left in place on the cervix up to 24 hours, during which time the patient is able to leave the hospital and continue with her daily activities, before removing and disposing of the device herself.
The clinical trials will investigate this advanced form of photodynamic therapy in patients with cervical precancer as an alternative to current surgical procedures, such as laser therapy, surgical conisation, LEEP excision or cryotherapy (freezing). These can damage healthy tissue and cause long term health issues including post-surgical infections, reduced fertility and an impeded ability to carry a child full-term. The study will also investigate the suitability in treating patients with mild cervical abnormalities, as this new approach could be an alternative to the numerous and stressful follow-up examinations patients currently have to endure.
'This is the first ever non-surgical treatment for HPV and precancerous lesions of the cervix to be successfully developed,' comments Dr Peter Hillemanns, professor and chairman at the Department of Obstetrics and Gynecology of Medical University Hannover, and principal investigator for the trial. 'If this sophisticated and breakthrough device is accepted, it will make way for a new era in cervical cancer treatment that will not only minimise patient risk and suffering, but could also help reduce the burden of HPV on healthcare systems.'