The speed of chemical reactions vary immensely – compare a rusting nail to dynamite exploding. At an atomic level though, breaking and reforming bonds happens very quickly indeed, on the order of picoseconds (10-12s) and femtoseconds (10-15s). To study events occurring over these timescales requires highly specialised equipment, not least ultrafast lasers with femtosecond pulse durations.
In the early 1990s, Richard Mathies and his team at University of California (UC), Berkeley, demonstrated that the photoisomerisation of the 11-cis-retinal chromophore in the visual pigment rhodopsin took 200fs, which, at the time, was one of the fastest chemical reactions ever studied. Photoisomerisation of the 11-cis-retinal chromophore, caused by the absorption of a photon, is the main photochemical reaction in the rod photoreceptor cells in our eyes. This photon absorption causes a cascade of conformational changes in the surrounding protein that excite the retinal rod cell to enable vision.
Later, in 1999, Professor Ahmed Zewail at the California Institute of Technology won the Nobel Prize in Chemistry for his work on femtochemistry and studies into the transition states of chemical reactions using femtosecond spectroscopy.
Today, Mathies continues studies on the photochemistry of vision using a range of time-resolved spectroscopic techniques including Femtosecond Stimulated Raman Spectroscopy (FSFS), a technique his team have developed that provides a time resolution of less than 50fs.
Transient absorption spectroscopy
Groups studying the photochemistry of vision and other chemical reactions at an atomic level typically utilise transient absorption spectroscopy. The technique covers a broad range of time-resolved spectroscopy techniques, which are all fairly similar in the sense that they use two beams, one to pump and one to probe the sample. The pump pulse excites the molecule to a higher energy state, which is then examined with the probe pulse to determine how the molecule changes over time. Transitional states of the molecule will exhibit a characteristic spectral fingerprint.
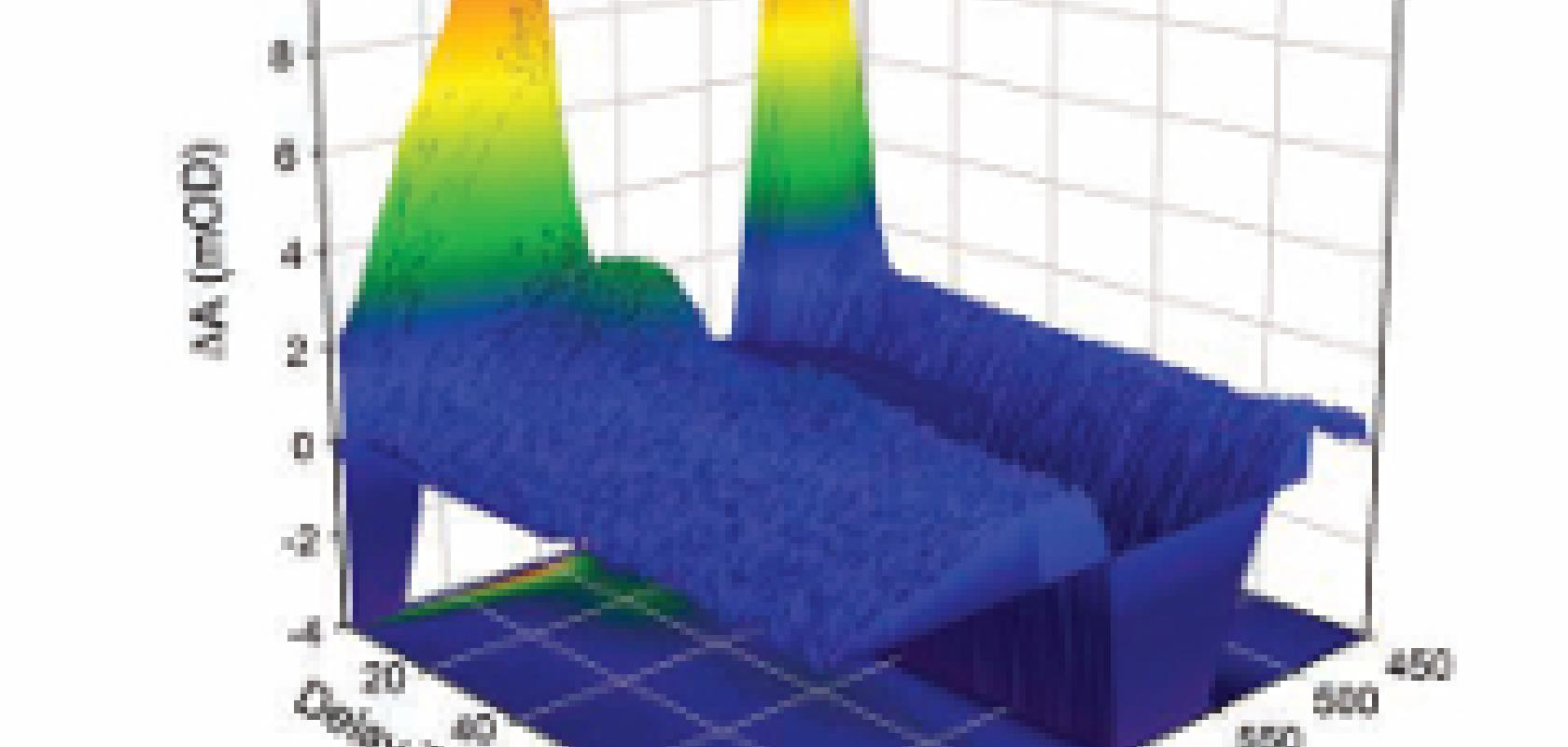
One of the requirements for many scientific studies using transient absorption spectroscopy is to make the measurements quickly. Dr Pancho Tzankov, R&D manager and senior scientist at Quantronix, comments: ‘Within minutes you have to be able to acquire all the spectral data from a sample.’ Many samples, especially in life sciences, decay rapidly over time and researchers want to acquire spectra as quickly as possible before they decompose chemically. ‘An accurate temporal readout requires at least 100 sampling points with sufficient signal-to-noise ratio [<0.3mOD]. Researchers typically want to make these absorption measurements in less than 10 minutes,’ continues Tzankov, adding that, in the past, transient absorption spectrometry took days to complete a scan with the same signal-to-noise ratio.
Quantronix, with headquarters in New York State, manufactures spectroscopy equipment. Its transient absorption femtosecond spectrometer is called InSpect-TA, which integrates an Avantes spectrometer as its detector.
Current pump-probe spectrometers use a white light continuum (WLC) probe pulse, which probes all the wavelengths of interest, typically in the visible region from 450 to 750nm, (Quantronix also provides systems covering 350 to 2,400nm). ‘Using a broadband continuum revolutionised the field of pump-probe spectroscopy, allowing researchers to track all the energy redistribution within a molecule,’ states Tzankov.
The pump pulse is generated by an optical parametric amplifier (OPA), producing femtosecond pulses that can be tuned to the required wavelength. Quantronix integrates its own OPA, called Palitra, into InSpect-TA.
‘Avantes spectrometers have optical coatings that sort light according to diffraction orders, thereby ensuring each wavelength is imaged to a separate pixel on the detector,’ Tzankov says. ‘With a WLC for the probe pulse, you have to ensure each wavelength goes to a separate pixel to avoid artificial contamination of the measured signal.’
The Avantes spectrometers contain around 2,000 pixels over the spectral range, which allows a high speed of acquisition.
Timing is everything
The temporal resolution is defined by the duration between the pump and the probe pulse. The shorter the time difference between the two pulses, the faster the reactions that can be investigated.
Transient absorption spectroscopy uses laser pulses on the order of 35fs or less. Other applications, like time-resolved Raman spectroscopy, use longer pulses of a few picoseconds to match the spectral resolution of the Raman transitions.
The Femtosecond Stimulated Raman Spectroscopy (FSFS) technique Mathies is developing at UC, Berkeley, combines picosecond and femtosecond pulses to get both a narrow spectral resolution and also a high temporal resolution. ‘In one sense, this almost breaks the time-bandwidth limitation,’ comments Marco Arrigoni, director of marketing at Coherent, which suppliers OPAs and lasers for time-resolved spectroscopy. ‘Typically, Raman spectroscopy needs a resolution of one or a few wave numbers and this means the pulses have to be in the picosecond range. Therefore, a phenomenon that takes place on a timescale of 100fs cannot be studied with standard time-resolved Raman spectroscopy.’
The challenge with FSFS, according to Arrigoni, is how to get both picosecond and femtosecond pulse durations at the same time. One way is to split the pulses in two and then narrow the bandwidth of certain pulses through spectral filtering, he says. Alternatively, Coherent supplies a second harmonic bandwidth compressor, which is a variation on standard OPAs. This device is able to convert a femtosecond pulse into the second harmonic and then rearrange the spectrum so that it can pump a picosecond OPA.
A second variation on standard pump-probe spectroscopy is multi-dimensional spectroscopy, which uses three laser beams, rather than two, to excite the sample. ‘What multi-dimensional spectroscopy allows you to do is analyse, not only a molecule’s excited states, but how excited states of neighbouring molecules cross-link between each other,’ explains Arrigoni. Conventional pump-probe spectroscopy provides information on how a known excited state interacts with the rest of the molecular system; multi-dimensional spectroscopy gives information about the cross-links between different excited states. This is particularly useful for studying complex molecules such as proteins.
Typically the three beams are obtained from a single laser source, split and temporally delayed with respect to each other. ‘The complexity of the instrument setup arises because the beams have to arrive at the sample with a temporal delay of interferometric quality,’ states Arrigoni.
Plasma studies
Other time-resolve studies occur at a slower pace. Laser induced breakdown spectroscopy (LIBS), for instance, uses a focused laser beam to atomise a portion of the sample and form a plasma. The light emitted from the plasma as it cools can be captured as a spectral fingerprint. The atomic emission lines are characteristic of the elements within the sample, and can be used to identify the chemical composition of the material. ‘The plasma is short-lived, lasting a few hundred nanoseconds or less, so this is very much a transient process,’ explains Gerald Cairns, applications specialist, spectroscopy at Andor Technology.
Andor offers intensified CCD (ICCD) cameras suited to these types of studies occurring over nanosecond timescales. ‘The intensifier of an ICCD camera can be triggered precisely to control the delay and exposure time necessary to acquire the spectrum,’ Cairns says. This is important to avoid the initial bright spark of light from the plasma that would mask the emission spectra; there needs to be a tightly controlled delay in acquiring the signal to discern atomic emission lines.
Other time-resolved spectroscopy techniques, including pump-probe spectroscopy, can use a standard CCD camera or an EMCCD, in fast kinetics mode, according to Cairns. The temporal resolution is determined by the pump and probe laser pulses and the pulse triggering control, rather than the CCD detector. Fast kinetics mode allows the camera to operate in a burst mode to read out the signal as quickly as possible and capture a string of spectra over time.
Moving to attoseconds
Other time-resolved spectroscopy techniques include terahertz pump-probe spectroscopy and attosecond studies. Terahertz radiation (around 50 to 100µm wavelength) is generated using high repetition rate and high-power amplifiers, typically 10W and 10kHz to give 1mJ/pulse, according to Arrigoni. ‘This is a developing technology that’s been around for a number of years, but we see a good market with high sales of amplifiers,’ he says. ‘Every material has a terahertz spectrum, just as it has a Raman spectrum.’
Attosecond spectroscopy achieves a very high temporal resolution. ‘Attosecond probe pulses allow studies on the motion of electrons,’ says Arrigoni. ‘Femtosecond pulses allow you to freeze vibrational motion, but with attoseconds you can freeze electron motion.
‘To generate attosecond pulses, requires a very short pulse to begin with,’ Arrigoni continues. ‘Coherent provides lasers that generate 25fs pulses.’ High energy per pulse, in the order of 1 to 5mJ, is also necessary. The ability to freeze electron motion opens up a whole new area of study with regards to the dynamics of molecules.
Fluorescence has grown from a specialist technology in the 70s and early 80s to one of the dominant methodologies in the biological sciences. Time-resolved fluorescence spectroscopy takes advantage of the time dependence of the emission. ‘The fluorescence decays are independent of total intensity of the fluorophore and you can learn things from the decays that you can’t learn from the steady state intensities,’ comments Professor Joseph Lakowicz, director of the Center for Fluorescence Spectroscopy (CFS) at the University of Maryland, School of Medicine.
In biophysics, for instance, the emissions from labelled proteins changes over time depending on whether they are folded or unfolded, or if they are associated with other molecules. Professor Lakowicz comments that the typical lifetimes of fluorophores are in the region of 2-3ns. CFS sources equipment from various companies, including from Berlin-based PicoQuant, which develops time-resolved electro-optical instrumentation used to make measurements on these timescales. ‘Pulsed diode lasers, such as those from PicoQuant, now provide resolutions in the order of tens of picoseconds,’ says Professor Lakowicz.
Professor Lakowicz and his team are investigating coupling fluorophores with metals to channel the fluorescence emission in a single direction and improve the signal. ‘Fluorescence imaging is reaching its natural limits,’ he states. ‘The detectors are highly efficient; the lasers can give you just about anything you want; there are now numerous fluorescence probes available; and everything you do is diffraction limited to around 300nm.’
A nano-structured, thin film metal like silver, gold, or aluminium coupled to a fluorophore will serve as both a substrate for enhancing fluorescence, giving it directionality, and diffracting background light at the same time. ‘This is all possible because it’s near-field effects, rather than light radiating, hitting the grating and being diffracted – it’s a direct coupling,’ explains Professor Lakowicz. ‘You can make single molecules much brighter and resistant to photo-bleaching.’
One area where this will potentially be applied is in medical diagnostics. Using fluorophores with metallic nano-structures would simplify the assay, according to Professor Lakowicz, and would make it cheaper, which potentially allows it to be carried out at ‘the bedside’.
‘In the future we’re going to see digital clinical assays,’ Professor Lakowicz continues. ‘There are limitations now that prevent single-molecule detection in flowing samples or anything with a background solution – we need to amplify the signal to detect it. I think these plasmonic structures are going to allow the creation of relatively simple metallic substrates that will allow you to count individual molecules as they flow through a volume. You would then have the ultimate sensitivity – you wouldn’t need to do any amplification to detect the signal.’