Increasing spatial resolution in fluorescence microscopy generally involves spatial constraints on the illumination field. In so doing, losses in the microscopy light budget are incurred, which must be compensated for with increased output from the light source. For example, super-resolution microscopy by single-molecule detection localisation is capable of a 10-fold increase in spatial resolution compared with widefield fluorescence microscopy; however, it requires irradiance (power/unit area) on the order of 104 mW/mm2.
Lumencor’s CELESTA and ZIVA light engines are designed to meet and exceed even these very demanding illumination requirements. Both house an array of up to eight individually addressable solid-state lasers within an optics and electronics control framework. Their many capabilities are assembled in a compact, turnkey, bench-top device (Figure 1, pictured above). Laser outputs are spatially coincident, so the illumination pattern does not change when output is switched between laser lines (Figure 2). This is a critical characteristic in multicolour fluorescence imaging using sequential illumination from two, three or more laser colours.
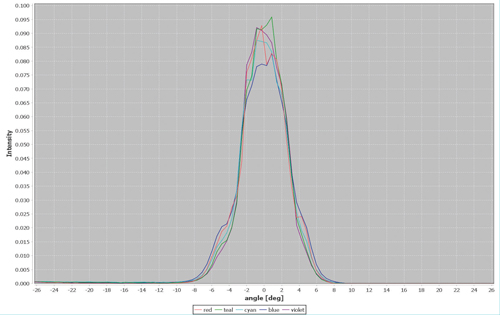
Figure 2. Spatial distribution of the 405nm (violet), 446nm (blue), 477nm (cyan), 518nm (teal) and 637nm (red) outputs from a 100µm diameter, 0.1 numerical aperture optical fibre coupled to a ZIVA light engine
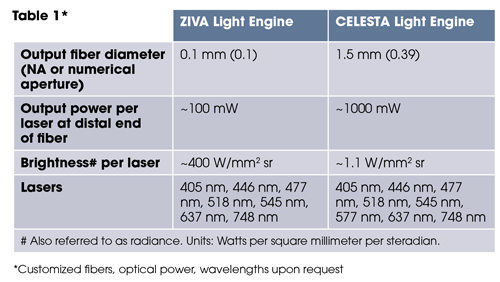
The laser outputs are merged into a common optical train passing through an onboard metrology module and a despeckler to the light output port on the front panel (Figure 1). The ZIVA and CELESTA light engines are distinguished by optimisation for coupling into various optical fibres (Table 1, below). This dictates the suitability of the ZIVA and CELESTA light engine for different applications.
The output of the ZIVA light engine is well-suited to structured illumination microscopy (SIM), stochastic optical reconstruction microscopy (STORM; Figure 3) and other super-resolution microscopy techniques. In these applications, ZIVA provides an alternative to more costly and hard-to-align laser sources. The larger illumination fields produced by the CELESTA light engine are preferred for applications such as spinning disk confocal microscopy (Figure 4) or MERFISH. MERFISH is an imaging technique that profiles cell populations based on identification of thousands of RNA transcripts per cell[1]. Figure 1. A CELESTA light engine, overall dimensions and light output port Identification is based on barcodes that are cumulatively assembled during multiple cycles of imaging and sample processing.
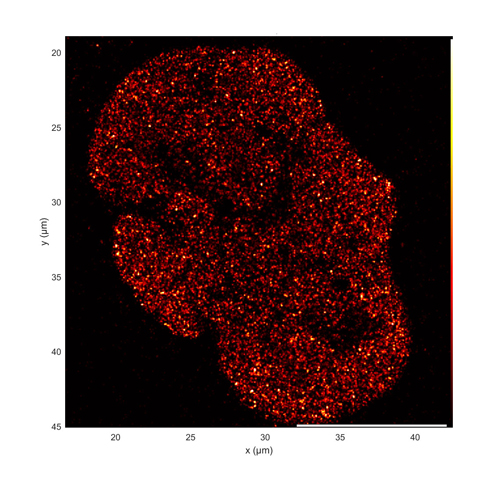
Figure 3. Super-resolution dSTORM image of the distribution of acetylated histone H3 (H3K27ac) in a single U2OS cell nucleus. H3K27ac is a marker for sites of active transcription. Image acquired using simultaneous 637nm and 405nm illumination from a ZIVA light engine. Image courtesy of Jan-Hendrik Spille, University of Illinois at Chicago
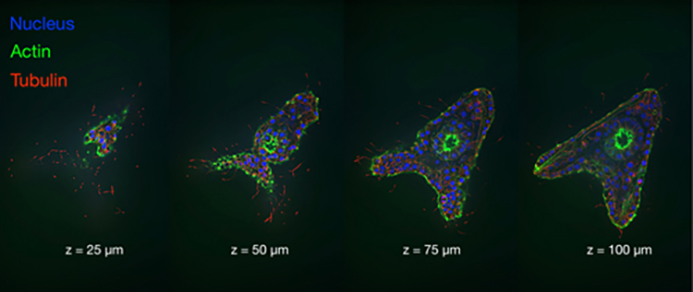
Figure 4. Four vertically spaced optical sections of a late blastula stage sea urchin embryo. Images acquired using a CELESTA light engine and a CrestOptics X-Light V2 spinning disk confocal scanner. Images courtesy of John Allen, Nikon Instruments, In
Acquisition of a complete data set may take several days[1], placing a premium on light source output stability. MERFISH requires a high intensity, spatially uniform illumination field matched to the dimensions (~200mm2 ) of typical sCMOS camera sensors. To meet this requirement, a critical epi-illuminator coupled to the fibre output from the CELESTA light engine is installed in the microscope, providing about 500 mW/mm2 at the sample plane.
Reference
[1] JH Su, P Zheng, X Zhuang et al. Cell (2020) 182:1641–1659


