The Covid-19 pandemic put sophisticated technology once confined to a laboratory into the hands of the public, and point-of-care (POC) testing became an everyday experience.
From MRSA to cancer, the lack of fast and accessible diagnostic tools results in millions of preventable deaths each year, but photonics-based solutions aim to change this – by delivering effective testing of numerous illnesses quickly and cheaply at the patient’s side.
Photonic-based POC systems have progressed rapidly in recent years, thanks to the advance in photonic integrated circuits (PICs), which enable the miniaturisation of optical technologies already used in medical diagnostics in a scalable – and therefore affordable – way.
A range of PIC innovations are being developed that could revolutionise how patients are diagnosed and treated, yet there are hurdles to overcome until we see these devices in hospitals – from finding the right commercialisation partners to changing society’s attitudes towards new healthcare technologies.
Tackling antibiotic resistance
One POC device aims to tackle the growing threat of antibiotic resistance. Dutch start-up Nostics has developed a handheld system that combines surface enhanced Raman spectroscopy (SERS) chips with nanotechnology and AI to diagnose bacterial and fungus infections in minutes, without having to send samples off to a laboratory.
The crucial benefit of the device is its ability to identify the exact species of bacteria or fungus causing an illness. This allows doctors to determine whether antibiotics are actually needed, and which types will be most effective at treating specific strains. Broad-spectrum antibiotics are often used today in the case of suspected bacterial/fungal infections – simply because identification takes too long or is simply not possible. Preventing the overuse of broad-spectrum antibiotics is a vital part of tackling resistance, which the World Health Organisations labels as one of the greatest threats to society today.

Nostics’ SERS platform has been combined with AI to diagnose at the point of care (Nostics)
Nostics’ device is based on SERS, whereby novel nanomaterials deposited onto the surface that interacts with the sample make the Raman signal about one billion times stronger. This leads to much more pronounced peaks in the sample’s spectrum, which through pattern recognition can be matched to the spectrum of the exact species of bacteria or fungus in a database.
A removable, specially-developed cartridge above the spectrometer filters the sample – for example, blood or urine – which is then analysed by the Raman spectrometer, and the spectral data is sent to the cloud to analyse the results. A dashboard on a smartphone, tablet or laptop allows non-experts to instruct the device or view results.
The company’s technology has been used to identify fungal pathogens such as Candida auris, a drug-resistant potentially deadly fungus that hit news headlines recently due to its spread across hospitals in the United States. In preliminary experiments, Nostics’ technology detected and distinguished different Candida species from culture plates, including C. auris, and the company is now investigating the platform’s potential to be developed into a product that could quickly identify the pathogen in hospitals to improve surveillance and prevent future hospital outbreaks.
Choosing the right spectrometer
Nostics’ system uses an off-the-shelf spectrometer and, while a number of spectrometers on the market would have been compatible, certain capabilities were required, explains Eva Rennen, Co-Founder & COO: “You need to make sure that every single time, your product works. It can’t happen that there is a risk of the spectrometer producing different results, for example if the spectrum is influenced somehow, or the calibration is not up to par.
“You need to mitigate every single risk that might cause a wrong diagnosis to happen. So we always test multiple batches of spectrometers multiple times, and we will put them under stress. We want to make sure that when someone drops it, for example, you can detect that the drop has occurred and if something has been broken, or the spectrometer is just so robust that nothing happens.”
Rennen added that SERS is still a fairly novel technique on the market, and so testing the data that the available products produced was a factor. Nostics also developed its own software to meet the demands of their application.
The company is currently working on getting regulatory approval for its handheld device, which in Europe comes with challenges, particularly as the region is quite fragmented.
“You would think that if you have a CE mark, you can market your device across Europe, but that’s not really the case,” Rennen highlights. “There are country-specific health technology assessments, and you have national regulations. Sometimes, even though they’re not official regulations, it is still common practice to have to do certain tests whenever a new medical device is coming to market in certain countries.”
There is also a diverse set of healthcare systems that exist across Europe, which companies need to familiarise themselves with to integrate their products into medical settings.
“Having so many different countries and cultures in Europe is normally great, but it is also difficult, because every single time you need to make sure you completely understand the healthcare system and reimbursement landscape,” says Rennen. “I think having more European-level regulations, policies or guidelines for the use of antibiotic diagnostics, specifically in AMR [antimicrobial resistance] is going to be extremely important.”
But tackling resistance to treatment also requires a huge shift in the way we view and accept the use of antibiotics in society, Rennen says: “We as a society need to change our way we use and administer antibiotics. And that requires a societal change in terms of understanding that antibiotics will become less effective if we don’t change the flow of infection to treatment.
“We want to make sure that no antibiotic is prescribed without an accurate and data-driven diagnosis, and this requires a systemic change of physicians and patients being open and accepting of new diagnostics,” states Rennen.
Healthcare systems and insurance companies also need to get on board to aid adoption, Rennen adds. “If we don’t invest in diagnostics now, it will result in issues in the future, and those kinds of health economics need to be in place. I think that’s not just for us, but it’s really for any diagnostic company looking at AMR to contribute to preventing antibiotic resistance. That’s one of the bigger challenges.”
Nostics is finding partners to help progress the various aspects of getting a new medical technology into healthcare settings. “We strongly believe on all levels that collaborating both for distribution or commercialisation is clever,” says Rennen. “We have a lot of options open.”
Another organisation developing photonics tools to tackle antibiotic resistance is the Leibniz Institute of Photonic Technology in Jena, Germany. The institute investigates a range of POC diagnostics, but has recently developed a test that can detect genetic characteristics of the bacterium Staphylococcus aureus and distinguish it between more than 700 strains.
The microarray test kit detects genes using the microarray hybridisation method. Several capture molecules are applied to a biochip in a miniscule space, to which DNA fragments from a bacterial culture can bind. If such a binding occurs, the corresponding field on the chip changes the colour and can be read optically. Based on the resulting patterns, the researchers know immediately which genes are present. This insight can then be used to determine, for example, if several isolates from an intensive care unit match, which could point to an outbreak.
The test is currently being used in clinical trials, and work has been carried out to validate a rapid test for the detection of the bacterial toxin, PVL.
Sensitivity gains with bio-coatings
Surfix Diagnostics has created a photonic sensor that can detect multiple biomarkers at the same time, thanks to an innovative coating applied to the chip.
The photonic chip design consists of six asymmetric Mach Zehnder interferometer (aMZI) sensors with a spiral layout that increases the sensing area. The light travels through the spiral-shaped structure, comparable to a miniature optical fibre.

Artist impression of Surfix’s photonic biochip with receptor molecules (green, Y-shaped) on the spiral-shaped structure and biomarker molecules (orange spheres) (Surfix Diagnostics)
Receptor molecules coated onto the surface of the photonic biochip can selectively catch and bind specific biomarkers in a sample, based on biorecognition. The interaction between the receptor molecules and the biomarkers causes a change in the properties of the light travelling through the chip. This change is detected and then translated into a useful diagnostic result.
The Surfix biosensor combines the photonic biochip with a microfluidic cartridge, which processes the sample before sending it to be analysed on the chip.
The technology can achieve an intrinsic sensitivity of 10-8 RIU, and this is largely thanks to the nanocoatings on both the sensor chip and cartridge, which enhance the sensitivity of the sensor and the flow of the sample, and reduce unwanted binding of biomolecules. A spin-off from Wageningen University & Research in The Netherlands, Surfix was originally a coating company before deciding to focus fully on the POC solution it currently offers after adding biosensing, microfluidics and (system-) engineering experts to its team.
According to Dr Sandor Snoeijers, the company’s CCO, the team is developing partnerships with companies that will integrate the device into a POC product, as well as apply for regulatory approval and work on commercialisation. The major demands from medical device manufacturers, Snoeijers adds, are high-sensitivity (to reduce the amount of sample required), high-specificity, and the ability to detect multiple biomarkers.
One of its customers is developing a screening system to measure cancer biomarkers in urine, in one of the many “liquid biopsy” applications the device could be used for.
While the medical device manufacturer will market the whole device, Surfix produces the chips, cartridges and readout systems, which it has manufacturing partners for but is currently looking at options for scaling up.
Having standardised processes for chip manufacturing could help companies such as Surfix achieve this, Snoeijers highlights: “There are a lot of companies that have various chip requirements. And if everyone is manufacturing chips for their own purpose, it is not very efficient. I think standard processes might help to make the chips cheaper through economies of scale.”
Integration of bottle resonators
The Fraunhofer Institute for Reliability and Microintegration (IZM) is also seeking partners to scale up a chip-based biosensor it developed as part of a transnational project (PoC-BoSens).
The sensor is able to quantify the biomarkers for a number of infectious diseases in less than 15 minutes. To achieve this, the chip includes a “world-first” combination of photonic bottle microresonators with microfluidics (to channel the sample).
Bottle microresonators are highly sensitive and compact, but a lack of suitable integration solutions for them, or other three-dimensional optical microresonators made with optical fibres, has impeded their use as biosensors.
PoC-BoSens’ biosensor prototype represents the first successful integration of four bottle microresonators in a practical cartridge. Using the semi-automated tools available in the Fraunhofer IZM labs, the microscopically small structures could be placed on a photonic chip, an operation that demanded extreme precision at a scale of less than a single micrometre. The hybrid photonic chip was then coupled with a microfluidic chip in a combined optofluidic design that is ideal for multiplex molecule detection.
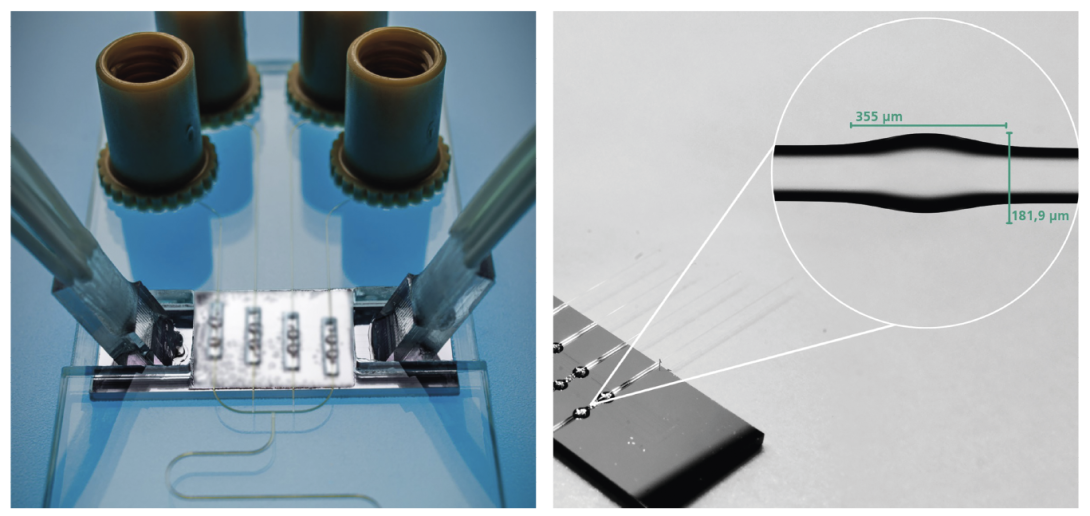
The PoC-BoSens project’s optofluidic cartridge for detection of cytokines (left) and photonic chip with four bottle microresonators (right) (Fraunhofer IZM | Volker Mai)
The cartridge chip was then developed together with a readout system to complete the PoC-BoSens platform, which has already demonstrated its capability in analysing cytokines, a group of proteins that play an important role in the human immune system and certain infectious diseases like tuberculosis or Q fever.
The system can also be used to test for antibodies that need to be identified and diagnosed quickly in certain medical conditions like celiac disease.
With the team overcoming a key challenge in integrating the bottle resonator components, the task now shifts to producing this at scale. A key part of this is to fully automate the assembly process, according to Dr Alethea Vanessa Zamora Gómez from Fraunhofer IZM, who coordinated the project.
“We had a lot of skills or competencies in assembly, and learned a lot in assembling lasers and micro optical systems. If we manage to fully automate the assembly process, this will take us closer to scaling up, but we need more funding and further partnerships to achieve this,” she said.
The semi-automatic processes the team developed during the project can be transferred to a new assembly machine, which could potentially make the process automatic, Gómez adds.
Along with Fraunhofer IZM, the project involved the Austrian Institute of Technology, Scuola Superiore Sant’Anna and Syel from Italy, German organisations MDX Devices, ifU Diagnostic Systems, Diarect, and Scienion along with Unitive Design & Analysis in the UK.


