How do you image an entire brain? It’s a question that has attracted millions of pounds of investment and stumped the world’s brightest scientists and industry leaders.
Yet, the field of neurophotonics is developing at a rapid rate. We are now entering an era where researchers can image portions of the brain while live subjects carry out tasks. One day, we may be able to image an entire human brain to unlock its inner workings.
However, as the complexity of the work increases, so must the complexity and functionality of the optical systems used in neurophotonics. Many researchers are leading this charge to produce optical systems that bring us one step closer to imaging the brain.
‘It is a new and rapidly-evolving discipline, so it’s been crucial to work in collaboration with the leading scientists to develop the correct solutions to address this market,’ commented Dr Elizabeth Illy, director of marketing at Cobolt. Cobolt has worked in this field for more than eight years, and focuses on trying to simplify complex optical systems into single-box solutions, where the end user only needs to supply the modulation signal to use the equipment.
It’s in the blood
Professor David Boas, professor of radiology at Harvard Medical School, started measuring human brain activity with functional near-infrared spectroscopy (fNIRS) in 1997. This technique uses near-infrared spectroscopy with neuroimaging technology to measure aspects of brain function. This is often carried out to understand the relationship between activity in certain brain areas and specific mental functions.
Using fNIRS, brain activity is measured through the rapid delivery of blood to active neuronal tissues of the brain – a process also known as a haemodynamic response. Professor Boas said: ‘My colleagues were studying cerebral physiology and pathophysiology in animal models, and I realised we could make new instruments to help them address outstanding questions about haemodynamics within the brain. We have continued to develop more neurophotonic methods for quantifying oxygen delivery and consumption within the brain, in health and disease, and its relation to neuronal activity.’
Understanding how oxygen is delivered to the brain through its dense network of micro vessels is important for better investigating neurological diseases, and interpreting signals from MRI scans. Many optical microscopy techniques exist, but multi-photon laser scanning microscopy (MPM) is the most mature and widely applied-for quantitative in-vivo imaging of the cerebral microvascular structure.
MPM is based on nonlinear optical processes, including two-photon excited fluorescence (TPEF). Here, fluorescence generation is essentially confined to the focal volume in TPEF thanks to its nonlinear nature, which means high resolutions can be achieved without the need for spatial filtering. And, as near infrared light is used – as opposed to visible light – less scattering and absorption in biological tissue occurs, which allows for deeper penetration.
Other methods include the use of laser speckle contrast imaging (LSCI). This is a powerful but simple tool for imaging blood flow in the brain in real time. Although measurements are limited to superficial tissues with no depth resolution, this technique has been used in pre-clinical studies of neurological diseases, and to understand the basic physiology of the brain. Professor Boas was one of the first to use laser speckle contrast imaging for imaging cerebral blood flow in animal models.
Another important development is the advancement of two-photon microscopy for mapping oxygen within the brain with micrometre resolution, using an oxygen quenched phosphorescent agent. Previous efforts faced difficulties because of extremely low two-photon absorption cross-sections of conventional phosphorescent probes. Recent research has allowed for two-photon high-resolution measurements by combining an optimised imaging system with a two-photon-enhanced phosphorescent nanoprobe, which opens up the potential for functional metabolic brain studies.
Getting the full picture
Imaging an entire brain is the primary objective for many current neurophotonics projects, but it is an elusive target.
Researchers at the University of Florence are pulling together experimental data to produce instruments that can accurately simulate the anatomy and functionality of mouse brains in aspects related to motion and rehabilitation after a stroke. Professor Francesco Pavone, professor of physics at the University of Florence and at LENS (European Laboratory for Non-linear Spectroscopy), explained: ‘In particular, we are working to characterise the spatio-temporal correlations of activation areas connecting this correlation with structural aspect. In this context, we are working to improve the clearing procedures, which are enabling the recording of a whole mouse brain with high contrast and resolution.’
The team recently optimised a custom-made light sheet microscope, to maximise image quality in terms of contrast, aberration corrections, speeds of acquisition and image analysis. Professor Pavone commented: ‘We increased the quality of images (in terms of signal to noise) by a factor 20, enabling the entire reconstruction of the brain vasculature, for example, and its segmentation.’
There are still many challenges to overcome when imaging large samples such as an entire brain, including the need to provide uniform illumination, control aberrations, give a high contrast and avoid image deformation. ‘High quality is a prerequisite to obtain quantitative measurements in terms of segmentations of shapes, or quantifications of structures,’ Professor Pavone added. ‘One the of the most difficult aspects in imaging is the quantifications of biological features. A sort of bio-metrology. In this respect, we are working on analysis tools that are able to work on big quantities of data and enable image treatment or quantification of image features.’
To preserve the image quality, the speed of image acquisition must also be improved. Segmentation helps, but this is a difficult task. A holistic approach is required, as Professor Pavone explained: ‘If you want to obtain, for example, the size of the blood vessels, their orientation, size, connections, and so on, in the whole brain, you will need to work not only on the images but also on the algorithms – so you must work with terabytes of data. So, all the neuro-informatic research, also in terms of data treatments and navigation tools, will be essential instruments to extract information from the brain.’
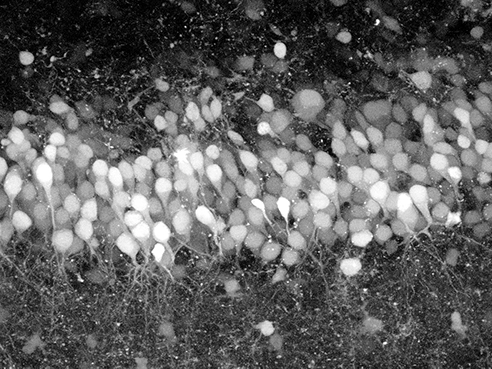
Hippocampal slice imaged using SLM microscopy (Credit: Columbia University)
Dealing with data
Analysing and simply coping with the sheer quantity of data produced by such neurophotonics research presents a huge computational challenge. It’s also a challenge that must be addressed to further the field of neurophotonics. The resulting knowledge will directly inform the models used by brain-modelling platforms such as SpiNNaker.
SpiNNaker is motivated by two primary self-fulfilling research questions: can we use the formidable computer power available today to accelerate our understanding of information processing in the brain? Can we use our increasing knowledge of the brain to produce more efficient, more reliable and more robust computer systems?
This research, along with the work at the University of Florence, forms part of the wider Human Brain Project (HBP) – a massive $1.3 billion EU-funded initiative to pursue an ambitious goal – building a simulation of the human brain. SpiNNaker will ultimately incorporate one million ARM (mobile phone) processors into a massively parallel architecture optimised for running large-scale real-time brain models. Steve Furber, ICL professor of Computer Engineering at the School of Computer Science, University of Manchester, said: ‘The current HBP platform is half this size. The unique feature of SpiNNaker is the way the processors communicate, which is optimised for real-time brain models – something that the fastest supercomputers cannot support.’
This work focuses around producing a computer chip that can meet the demands of the HBP. After five years and 40 person-years to design, the team produced a working chip, and has since used this to construct the 500,000-core HBP platform. Professor Furber added: ‘The real challenge now is to use SpiNNaker to generate new science. We have learnt a lot building the machine – and are now starting the design of SpiNNaker2 within the HBP – but the key goal is to deliver a new understanding of the brain and new applications based on that understanding.’
Brain regeneration
The regeneration of neurons in the brain forms part of the HBP’s research and Dr Anna Letizia Allegra Mascaro, a researcher at the National Research Council and LENS, is using different optical microscopy techniques to address different aspects of brain plasticity, which refers to the brain’s ability to change throughout its life.
Earlier research combined in-vivo two-photon and ex-vivo electron microscopy and allowed the visualisation of the integration of the newly formed neuronal branches into the circuitry of the brain under investigation. Dr Allegra Mascaro told Electro Optics: ‘Now my research is primarily focused on the use of in vivo imaging of different aspects of brain functionality to dissect how the brain reshapes due to robotic rehabilitation after stroke. In order to investigate complementary aspects of brain plasticity, we combine linear and non-linear fluorescence microscopy with optogenetics and behavioural analysis.’
The research is now combining new optics tools – like optogenetics – to foster the recovery of motor functionality after a stroke by using light-stimulation of neuronal plasticity. Dr Allegra Mascaro said: ‘This fascinating method, which allows modulating brain activity with light illumination, is proving to be a game-changer for disentangling the multi-level complexity of brain physiology and pathology.’

Cobolt 06-MLD 473nm used for excitement of opsins in behaviour studies
The 3D BRAIN
Imaging the brain requires a radical rethink of current microscopy methods and instruments, as Professor Rafael Yuste, professor of Biological Sciences and Neuroscience at Columbia University, explained: ‘2D microscopy is a technique with hundreds of years of development. But, if you want to image a 3D structure like the brain, then we need to redesign the microscope from scratch.’
The ‘Brain Research through Advancing Innovative Neurotechnologies’ (BRAIN) initiative was born at the UK’s Chicheley Hall during a brainstorming session on the future of neuroscience. Professor Yuste proposed a project, akin to the Human Genome Project, that would unlock the brain by instituting methods to measure and manipulate every neuron in the brain. This idea met considerable resistance, but four additional researchers banded behind it and proposed it to the Office of Science and Technology of the White House. President Obama endorsed it a year later, as the BRAIN initiative.
More than 200 labs are now involved in the BRAIN initiative, which received a further $70 million of funding in October from the US National Institute of Health. Professor Yuste said: ‘Neurophotonics is the central approach to the BRAIN initiative. While it is sponsoring many methods, the main ones are optical methods to read and write activity into the brain.’
This all-encompassing approach to imaging the entire brain, as opposed to a single neuron, is a marked change for neurophotonics, as Professor Yuste explained: ‘The method of recording the activity of neural circuits one neuron at a time is not ideal for trying to understand these systems. It’s like trying to understand a movie by looking at one pixel.’
However, research into the 3D imaging methods and indicators used to pick up single spikes of neuron activity is still in its infancy.
Current brain imaging techniques rely on calcium indicator dyes to measure neuron activity. This works by loading the neurons with a fluorescent dye that binds to calcium, and the brain activity is monitored using two-photon imaging of the fluorescence-tagged calcium.
Members of the Yuste Laboratory at Columbia University have extended this technique by incorporating a wavefront shaping technique using devices, called spatial light modulators (SLMs), to bend the light to stimulate and simultaneously image neurons in the brain, regardless of location. The result is, effectively, a hologram of the entire region of interest that combines recordings of the individual nerve cells and their spatial location in three-dimensional space.
In other words, you can now image the simultaneous activity of multiple neural populations in three dimensions. SLM microscopy can also be used for fast multifocal two-photon imaging, creating the potential for rapid deep-tissue imaging of multiple sites of interest.
Because SLMs can mimic many of the optical functions of a large microscope, it is also possible to miniaturise them to the point where they become portable, less expensive and used in future diagnostic work. The team is now working to improve its method to image large regions in the cerebral cortex of mice.
The BRAIN initiative is also investigating the use of a range of alternative voltage indicators, including nanoparticles with optical properties that respond to changes in an electrical field. This is a difficult undertaking, as the voltage indicators must pick up changes in the electrical field of the plasma membrane of a cell, which is only a few nanometres thick. Conversely, a calcium indicator detects electrical changes across the entire volume of a neuron, which is orders of magnitude greater.
The issues for voltage indicators do not end there, as Professor Yuste explained: ‘The voltage indicators need to be in exactly the right position for us to see a single spike of neuron activity. To make things worse, we cannot use as many molecules, as the plasma membrane is very sensitive to photodamage and high concentrations – or long exposure times – could kill the cell.’
These challenges, coupled with the fact that 3D microscopy techniques are still under development, mean imaging how the brain works could be a highly demanding problem. But there’s no need to despair, as Professor Yuste added: ‘Part of the problem is that, because the brain has a complex structure, people think it is a complex problem to resolve. But, just like the movie that we are currently watching with one pixel, we could reveal a simple movie if we could just see beyond the single pixel – just as the double helix revealed the simplicity of the DNA structure. I hope it will be something as simple and elegant as this.’
Neurophotonics is the key to unveiling this full panorama of the brain, as Dr Allegra Mascaro added: ‘Neurophotonics is a crucial milestone in understanding the basic mechanism of our brain, since it lets us dive into it with subcellular resolution, while still swimming over big networks.’


