Producing living tissue using a machine small enough to sit on a lab bench has long been limited to science fiction. But 3D printing, or additive manufacturing, has brought the Star Trek-style replicator dream closer to reality.
Now, refined developments in additive manufacturing have led to bioprinting, where cellular building blocks are deposited to form a structure. This technology offers long-sought solutions to the biomedical community.
‘The idea is like a MakerBot: a mechanical system that moves in three dimensions and extrudes cells,’ said Nadia Tsao, technology analyst at market research and consulting firm IDTechEx in Cambridge. But unlike a MakerBot’s spools of polymer filaments, bioprinters use reservoirs with a low-viscosity biological polymer known as bioink.
From printers that extrude a bioink and cure it carefully with UV light, to those that transfer single cells using a laser – bioprinting tools are driven forward by developments in photonics that bring pre-clinical biomedical testing closer to important results.
‘In early days, they looked at commercial inkjet printers and modified that to empty out ink; feed in cells,’ Tsao observed. ‘But now we have printheads specifically designed for biological functions: milder conditions improve the outcome.’
The bioprinting market is set to reach $1.8 billion by 2027, as companies founded within the last five years are creating products. Now, they’re looking for companies to pick up on the technology, according to Tsao.
Making bioprinting affordable
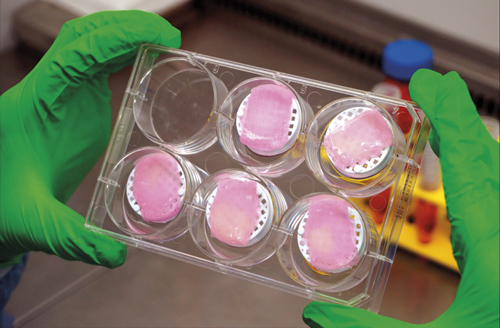
To print a tissue, you need a bioink and a desktop-sized printer. Boston-based biotech company Cellink offers both, referring to themselves as the ‘first bioink company in the world’. They provide sterile and ready-to-use inks, and collaborate with scientists to develop new varieties. Bioinks are based on polymers such as collagen, gelatin, hyaluronan, silk, alginate, and nanocellulose. They provide an aqueous 3D environment with chemical and physical properties that mimic the natural extracellular environment, providing a temporary support to cells which, in turn, produce their own extracellular matrix.

Cellink's BIO X printer features three independently controlled, interchangeable printheads that allow for numerous combinations of printing parameters. (Credit: Cellink)
Cellink built its second generation BioX Printer using feedback from the initial Inkredible model. The BioX features a filtration system that provides a clean chamber environment for sterile printing without the need for a biosafety cabinet, and three independently controlled, interchangeable printheads that allow for numerous combinations of parameters as determined by the user. All printheads can record data on temperature, pressure, extrusion speed, and other metrics valuable for the user, and options include heated or cooled printheads, syringe-based extrusion for small volumes, inkjet printheads, HD camera, or special designs for the user’s custom application.
The printers incorporate LED lights that emit in a range of wavelengths for cross-linking bioinks, or inducing a covalent bond to form between molecules without damaging cells, so they retain their shape. ‘While cross-linking of materials via UV light is not unique to bioprinting, it is important in the field, because if a deposited filament or material cannot retain its shape after deposition, you cannot build up a bioprinted construct,’ said Patrick Thayer, Cellink’s chief bioink officer.
The company’s systems support a wide range of research and engineering applications. Many USA-based labs currently focus on developing cancer tissue models that can be used for developing better treatments. ‘By being able to print out hundreds of tumours, the researcher gets a great testing platform for new drugs, but also for understanding how the tumour develops and behaves,’ said Thayer.
He sees vast potential for the future of bioprinting. Needs include developing bioinks for specific tissue microenvironments, standardisation of bioinks to enhance reproducibility, developing bioprinters that can seamlessly integrate all bioprinting techniques into one system, and achieving nanoscale to tens of micron resolution in filament or droplet resolution.
Fully integrated systems
Another company addressing these needs is San Francisco- and Philadelphia-based BioBot. The company’s second version of its desktop bioprinting solution has six print heads, can print with precision to 100 microns, and uses auto calibration with a computer vision system. It measures 20 by 14 by 14 inches. The device pushes bioink from a reservoir through a nozzle, driven by a compressed air pneumatic system that makes it easy to start and stop a printing process. Then, LED lights in the visible to ultraviolet range do the key photocrosslinking process which hardens the material rapidly. Biobot specialises in photo cross linkable materials, a main category of interest for customers, although they do support a variety of other crosslinking methods.
The key to BioBot’s success, though, is the ‘marriage of software, hardware and wetware. It creates an entire solution, a new workflow for doing biology experiments,’ said its founder, Danny Cabrera, referring to the software tool that serves as a project management system for organising experiments and analysing data for any bioprinting project. ‘The beauty is scientists don’t need to worry about the printer. They can focus on the science.’ Starting with any CAD tool, a scientist can design the desired 3D geometry; upload the file; and the BioBot turns the file into print instructions.

The BioBot 2 printer is the most compact 3D bioprinter on the market (Credit: Danny Cabrera/Biobot).
Harvard University students are currently using the machine to help them develop a new model to determine how and when blood clots form in order to create new drugs. The BioBot was used to build a 3D blood vessel, in which they can induce blood clots under various conditions and observe microscopic tissue changes as fluid flows through the vessel.
In other cases, BioBot-printed tissues have been used to test new medicines; or for developing medical implants.
Single-cell resolution
The other bioprinting technique currently under development is known as laser-assisted bioprinting. Here, a laser deposits cells – or groups of cells – one by one onto a target, offering advantages such as the ability to deposit tiny quantities of cells in very specific locations; or to engineer cell monolayers. Poietis, based in Bordeaux, recently announced the NGB 17.03 bioprinter, which is the only laser-assisted industrial bioprinter currently on the market.
Sample of Poietis' bioprinted skin.
‘If you want high resolution, you need a very small diameter for the [printhead] nozzle. In that case, there are issues for viability of the cells, because you have a large shear stress on the cell membrane, and a large volume of cell death,’ explained Bertrand Viellerobe, chief technology officer at Poietis. His company achieves cell viability of above 90 per cent after printing, thanks to its patented laser printing technology.
The printer generates powerful laser pulses, directed at a sample of bioink. The bioink quickly absorbs the laser light, causing it to vaporise into a plasma. Then, plasma relaxation creates a highly pressurised droplet, which is then directed as a jet toward the substrate for printing. The entire process occurs within a sterile environment.
Each droplet is made to contain a specific number of cells, hence the high-resolution capability. ‘The laser interaction with the liquid relies on standard absorption processes, we don’t need anything special,’ said Bruno Brisson, the company’s VP of business development.

A spine cage manufactured by 3D metal printing (Credit: Concept Laser).
Poietis uses a layer-by-layer process to print additional extracellular matrix material, to ensure cells have the desired environment within the printed tissue. After printing each layer, the cells proliferate to yield the mature, functional tissue.
Poietis’ main application is printing skin tissues. Cosmetic and pharmaceutical companies want skin samples with specific parameters for testing; and Poietis plans to produce their own off-the-shelf tissue model for pharmaceutical research and cosmetic applications.
They’re also developing hair follicles, or the small organs that produce hairs, which are ‘biologically very complicated: tiny in size, with lots of types of cells,’ added Brisson. Cosmetics giant L’Oreal, a global expert on hair biology, collaborates with the firm to harness the single-cell capability of Poietis’ technology.
Looking forward, Brisson’s goal is to have a printer suitable for advanced medical development products for clinical applications. ‘Today, there is no bioprinter… available that is certified,’ he noted. This is a main challenge, because such an application would require biocompatibility, sterility, and reproducibility. He looks forward to proving Poietis’ ability to make the same product or tissue a hundred times in safe conditions.
Bionic men and women
Although bioprinting is still not established commercially, 3D printing holds a secure position in the production of biomedical implants. Concept Laser, based in Germany, has a business department dedicated to the medical industry. With their 3D printers and the suitable biocompatible material, Concept Laser customers can fabricate medical products such as dental prostheses which are unique for every individual patient; as well as hip, knee or maxillofacial implants for patient-matched solutions or off-the-shelf implants.
‘Our customers are interested either in standard parts produced in large volumes or patient-specific parts produced in small volumes. Our machines can do both,’ said Stephan Zeidler, business development manager for Concept Laser’s medical division.

A hip cup manufactured by 3D metal printing (Credit: Concept Laser).
3D printing for medical applications has changed from the idea of prototyping, to patient-specific implants, to creating medical products in mass production. To keep up with these needs, additive manufacturing has had to become more reliable and efficient. However, there is still a great potential for 3D printing in medicine and it will change the way of designing and manufacturing medical devices.
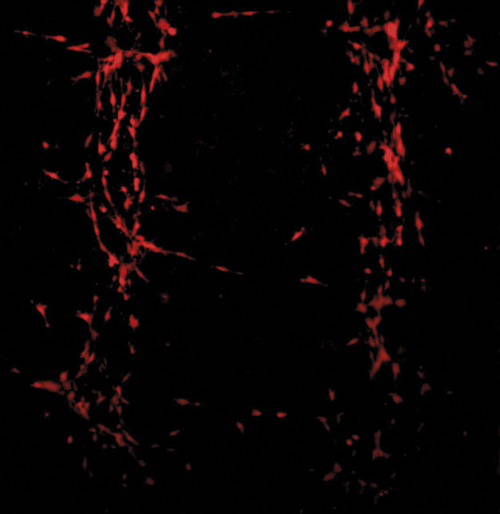
Poietis' imaging system allows users to monitor thie migration, proliferation, and differentiation of cells.
Concept Laser’s M2 Cusing machine uses selective laser melting to build a product from metal powders. The machine offers the option for 200 or 400W lasers: a 200W laser is suitable for smaller, more delicate parts; while a 400W laser is more powerful for running larger designs. Furthermore, it is possible to select between a single or dual laser option to increase the productivity of the printer by decreasing the build time.
Medical supply company KLS Martin uses Concept Laser’s M2 Cusing machines to produce patient-matched implants, which are designed from the patient’s MRI or CT scan data. Other clients seek mass production of a medical component, such as an off-the-shelf spinal cage for supporting a fused spine. Another example is medical device company Sutrue, which produces automated suturing devices and stabilising devices for keeping the heart muscle stable at a precise point during surgery. 3D printing makes the complex geometries of all of these possible.
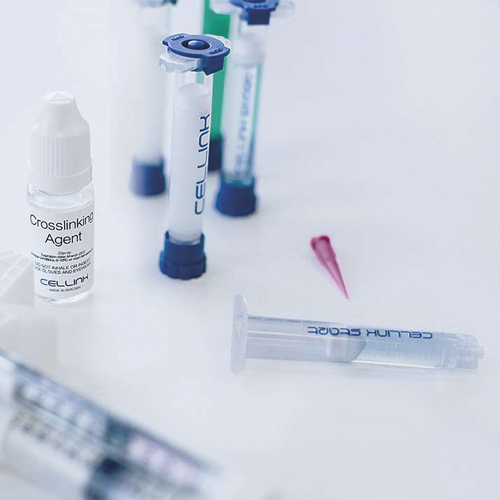
Bioinks available from Cellink.
‘The exciting part is that five years ago, the majority of 3D printers were installed in R&D departments or universities. Now, we’re talking about bringing hundreds of machines into factories and production lines for serial production, instead of using it for prototyping,’ said Zeidler.


