Light-based technologies are used widely in the cosmetics industry, from skin rejuvenation and anti-ageing procedures, to the treatment of vascular lesions and tattoo removal. However, as devices are becoming cheaper and more widely available, there is concern within the industry about the improper use of machines containing lasers or intense light sources. The concern will be a topic for a summit being held on the 9 December in London by Health Education England, which is working towards creating a formal qualification for users of light-based technologies in the cosmetics industry.
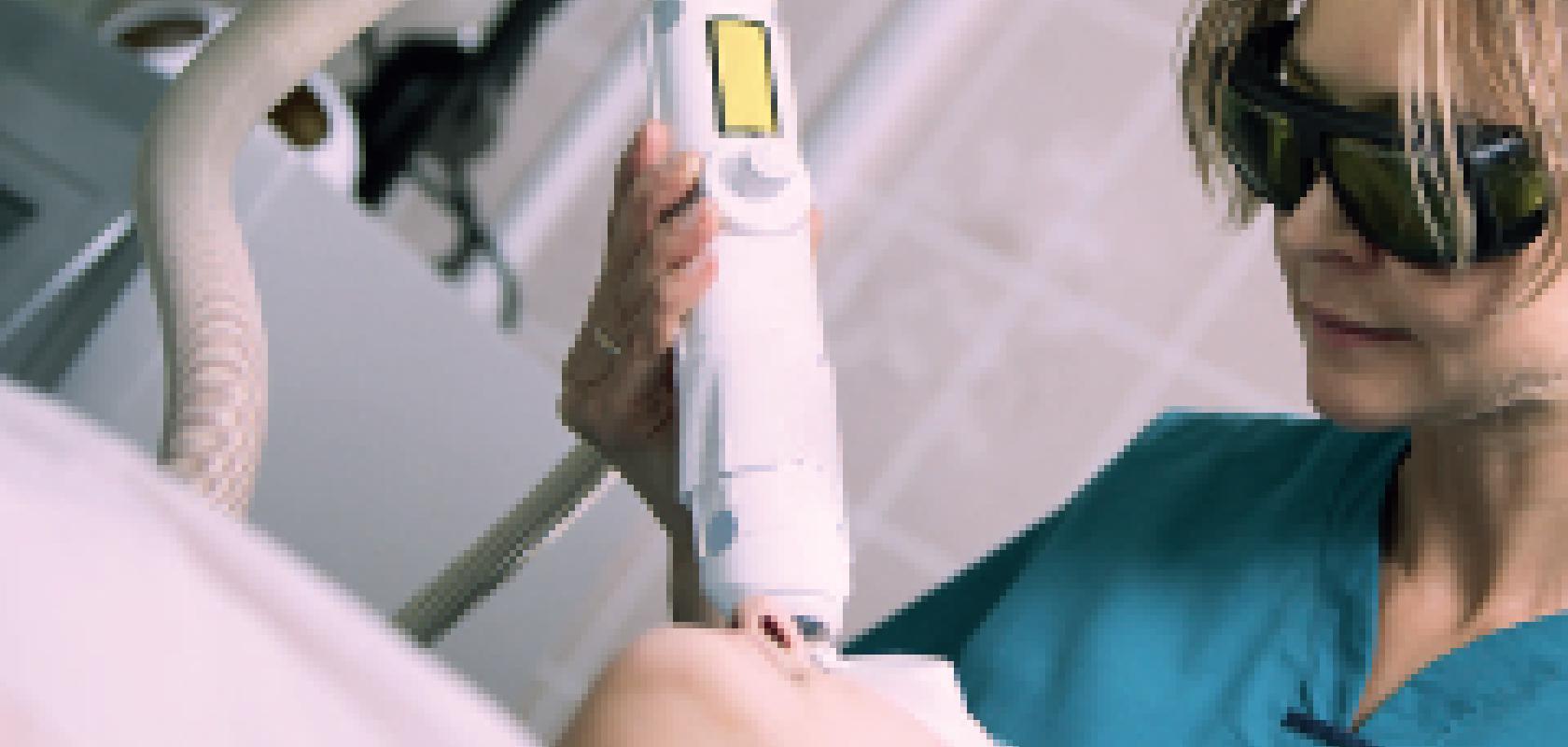
Popular cosmetic procedures include hair removal or the treatment of vascular lesions, which involves using a laser to create an effect known as selective photothermolysis. Here, the light energy is absorbed only by the chosen target in the skin, for example a hair follicle or blood vessel, and not the surrounding tissue. It is therefore essential to choose the correct wavelength of light to ensure that the reaction is confined to the target area.
‘If you choose a wavelength of light that is absorbed heavily by haemoglobin, for example, and not by the skin tissue, then that light can travel through the tissue until it hits the blood vessel,’ said Dr Jonathan Exley, managing director of Lynton Lasers in the UK. ‘It will then be absorbed by the haemoglobin, where the light energy will be converted to thermal energy and heat the target chromophores – and with sufficient energy in the laser pulse, the temperature of the target will reach a point where it will destroy the cells.’
Typically, for hair removal, the gold standard is to use a 755nm alexandrite laser, as the wavelength is absorbed by melanin − the pigment in hair − but not by the surrounding tissue, Exley said. Pulse durations in the millisecond range are used in most cosmetic applications to avoid damaging other parts of the skin. ‘It is important to match the laser’s pulse duration to the thermal relaxation time of the target, (e.g. a blood vessel) so that when the light is absorbed and turned to heat, there is minimal thermal conduction of that heat into surrounding tissue,’ he added.
Laser hair removal, according to Exley, is the application which ‘leads to lasers expanding from the hospital into salons on the high street’. Laser hair removal is becoming increasingly competitive, he told Electro Optics, while the market for tattoo removal and modification is growing at a rapid pace: ‘I think tattoo [removal] is probably the fastest growing application at the moment. In the early days tattoo removal was a very strong one, and then that started to decline in the mid-nineties – but over the last couple of years we have seen tattoo removal coming back again in absolute waves.’
Unlike the lasers used for hair removal and vascular lesions treatment which create a photo-thermal reaction, for tattoo removal, a Q-switched laser is used to produce a photomechanical effect. Tattoos are permanent because the pigment injected into the skin is too large to be cleared by the body’s immune system. However, by using a Q-switched laser, the light hits the tattoo pigment and rapidly heats it, causing the pigment to expand so quickly that it breaks up. This then allows macrophages and white blood cells in the body to attack and remove these smaller pieces.
As tattoos can contain many different colours, a range of wavelengths are needed to target each pigment, and so devices for this application often contain more than one laser to make sure that tattoos are completely removed. Lynton Lasers’ latest tattoo removal machine, the Q plus C, combines a Q-switched Nd:YAG 1,064/532nm laser and a ruby 694nm system. ‘The 1,064nm would be great for dark colours like blues and blacks, the 532nm is good for red colour tattoos, and the 694nm is ideal for greeny-blue tattoos,’ Exley said.
According to Exley, the market is also experiencing more of a demand for tattoo modification, whereby the tattoo is faded, or only part of it removed, to allow new tattoo designs to be added on top. Lower power passive Q-switched lasers with nanosecond pulse durations are often sufficient for modifying tattoos, rather than the active Q-switched lasers used for complete removal.
Safety first
With all devices that contain high-intensity light sources such as lasers, safety is always an issue. Health Education England (HEE) is currently working with the cosmetics industry to review the qualifications required for practitioners delivering non-surgical cosmetic treatments, and on 9 December in London, HEE is holding a summit to present a proposed educational and training framework.
According to Exley, who is also a member of HEE’s Expert Reference Group, the cosmetics industry would benefit from the introduction of an educational framework and indeed some form of better regulation. The machines used for tattoo and hair removal and other such laser treatments, can be purchased and used by somebody without receiving sufficient training. ‘Using inappropriate laser settings due to a poor standard of training could lead to an adverse reaction following treatment,’ Exley explained.
Recently, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) found that low-grade silicone, liable to rupture, was being used for a type of breast implant. Following the discovery, the government commissioned a review (the Keogh Review) into the cosmetics industry. Exley explained: ‘Within that review they identified that practitioners were using the equipment and they hadn’t necessarily had suitable training. One of the recommendations from the Keogh Review was that there should be some sort of formal qualification, and that’s why the Department of Health decided to ask HEE to review the qualification requirements for delivery of non-surgical cosmetic interventions,’ he said. ‘We’re currently trying to create a formal qualification for non-surgical cosmetic treatments for the use of lasers and light sources in this exact arena.’
Another safety concern is that some manufacturers are not making safe machines. ‘It’s now possible to purchase lasers via the internet which are then being used for cosmetic interventions. Many of these are supplied from overseas and are not necessarily built to the strict medical standards that a company like Lynton adhere to,’ Exley added. ‘Under these circumstances, there is no support from the supplier and the devices are of variable quality which is very concerning. My advice is to always look for a medically certified laser from a reputable supplier who can offer a high level of after-sales support.’
Testing safety
To make sure that safe products are being delivered to customers, companies are developing test equipment to ensure that instruments containing intense light sources are safe. In fact, a whole other market sector has been born from ensuring the safety of cosmetic equipment.
Test and measurement company Admesy is developing a system to test whether intense pulsed light (IPL) devices are safe, after being approached by a distributor of the devices who had concerns about the potential dangers of these instruments.
IPL devices work in a similar way to lasers used for hair removal, except an arc flash or a xenon lamp is used along with a dichroic filter that selects the desired wavelengths, and then the light energy reacts with the skin in a similar way to the laser. IPL systems can be used for treatments such as hair removal, acne treatment and red vein removal, but a growing application for the devices has been for anti-ageing, as they can deliver fast, unnoticeable effects through what is known as ‘lunchtime treatments’ – quick beauty treatments.
Just like with lasers, one potential danger with IPL devices is that if the intensity of the pulse is too intense it can cause burns, explained Steven Goetstouwers, CEO of Admesy. Another possible issue is that if the filter used to block out the UV light from the xenon lamp deteriorates over time, then the UV light could cause skin damage.
And, over the last couple of years, IPL devices have become more readily available and lower in cost, Goetstouwers added, which can also increase the risk: ‘You can see the handheld devices being sold for about two to three hundred Euros, online, or in general shops to the private consumer. The high-end devices are also more powerful,’ he explained. ‘[The distributor] wondered if we needed to calibrate the IPL devices occasionally, and the effect of multiple and intense use on the filter – if they deteriorate after a certain time frame.’
The system that Admesy has been developing to measure the intensity and duration of the light pulses is in the pre-production prototype stage, but it should be ready for launch next year. The current set-up consists of an integrating sphere to capture the light source, and, to analyse the signal, a high-speed light meter and a spectrometer containing a custom filter wheel for measuring UV light concentration.
As the company already produces light measurement systems for the display market, they were able to incorporate some of the same technology into the new IPL test device. To make incredibly fast measurements of pulse duration, taking advantage of this technology came in useful, Goetstouwers noted: ‘In the display market we used some light measurement equipment for the flicker measurement on displays, so this same equipment can do a very high speed measurement of the green light curve for characterising the [IPL] pulse and also to get the intensity of the pulse.’
Currently, the three devices exist together as a set-up, but once it is ready for commercialisation, they will be integrated into one instrument, according to Goutstouwers: ‘The idea is that we will bring it into one system, where on the top you have a spot to put the light source and the computer automatically shows all of the data.’
The company expects that there will be a variety of user groups interested in the measurement system. ‘We expect three types of users in the short run – importers that have a liability issue, specifically to countries which have a claim culture, such as America, where the importer might sample or 100 per cent inspect the items they get as a first step,’ Goutstouwers noted. ‘Large beauty parlour groups or chains might use it as a marketing quality aspect, so they can tell customers that they check all of the light sources regularly and are a safe company.’
In the longer term, the company expects that in the future, IPL devices will be required to have a medical CE check, which is needed for lasers used in the cosmetics industry. ‘We assume that in three to five years or maybe even sooner it will be a requirement [for IPL equipment] to have a medical CE, and then all of the producers, or at least the ones that want to import to western countries, will then have to do 100 per cent inspection,’ Goetstouwers said. ‘That is what we are hoping for the future.’
In Europe, all sunscreens marketed with a sun protection factor (SPF) or UVA protection factor (UVAPF) are required legally to be tested to see how well they protect against the sun’s rays. The in vivo test, or ‘on skin’ testing, involves exposing a person’s skin to a known level of artificial sunlight, and timing how long it takes to develop erythema – a reaction which causes the skin to turn red – with and without a particular sunscreen applied.
Pro-Lite Technology supplies solar simulation products from The Solar Light Company in America which are used for in vivo as well as for in vitro measurements on artificial materials. The company also supplies testing systems from Labsphere for in vitro testing.
Although every sunscreen product is still required to be tested using in vivo methods, the industry has been moving towards in vitro techniques, whereby SPF and UVAPF values are calculated by measuring the spectral transmittance on an artificial substrate rather than on human skin.
In 2007, the trade body for the European cosmetics industry, COLIPA, published a new set of standards for in vitro sunscreen testing. This prompted Pro-Lite and Labsphere to re-design their existing testing system, the UV1000, to match the then-new testing regulations, and it has been widely used by the industry ever since. ‘Both the UV1000 and UV2000 became the industry standard for instrumentation for measuring SPF and UVAPF,’ said Robert Yeo, CEO of Pro-Lite Technology.
‘I say that because, almost without exception, everybody in the European sunscreen and cosmetics industry uses our instrument – we developed it to meet this industry standard.’
According to the COLIPA in vitro method, sunscreen is applied to polymeric plates roughly 50mm2, which are then loaded into a sample compartment to ensure that all of the plates are exposed to the same level of radiation. As well as ensuring a good uniformity, it is important to make sure that the solar simulators only emit UV radiation so that the sample plates maintain their temperature. ‘A good solar simulator for in vitro testing doesn’t heat the samples, so the solar light system that we sell is designed with filters so as not to transmit infrared radiation through onto the sunscreen samples,’ Yeo said.
In the future, Yeo expects that sunscreens will be tested using only in vitro methods, to avoid damaging a person’s skin.