Neurophotonics, or the use of light-based technology to observe the brain and nervous system, has been advancing clinical research and treatments for brain disorders for the past few decades.
Advances have been made rapidly, with increasing interest – and therefore funding – over the last 20 years. The development of new-generation tools, techniques and other elements has moved the field on, but with still more to do, there has been no resting on laurels for the industry’s leading research groups and academics.
As the complexity of their work increases, so too must that of the optical systems used.
In the early noughties, the optogenetics technique was invented. This uses a combination of light and genetic engineering to control the cells of the brain. Optogenetics is now used in brain research laboratories all over the world. In recent years, neuroscientists have used it to study increasingly larger groups of neurons in animals such as rodents.
In this process, neurons are genetically engineered to express a particular protein marker, such as green fluorescent protein (GFP). The presence of GFP causes the cell to glow green when irradiated by blue light, providing a visual indicator of neural activity. By fusing sensor molecules with these markers, researchers can engineer neurons that signal their local activity by modulating this fluorescence.
Latest developments
One of the most recent developments in this area came from researchers at Caltech, who devised an advanced approach called integrated neurophotonics. They say that. with this technique, the activity of all of the thousands to millions of neurons in a particular brain circuit could be observed in real time. The work, detailed in a paper published in Neuron Journal, involved collaborators from 14 other institutions, and was funded by the National Institutes of Health (NIH) Brain Research through Advancing Innovative Neurotechnologies (Brain) Initiative grant, the Defense Advanced Research Projects Agency, the National Science Foundation, and the Kavli Foundation.
The Brain Initiative, launched in 2013, was set up as a large-scale effort to accelerate neuroscience research by providing scientists with the right tools and information to study a variety of brain disorders, such as Alzheimer’s disease, Parkinson’s, autism, epilepsy and traumatic brain injuries. Scientists have been developing tools for exploring neural circuits that underlie brain function since the initiative’s inception.
Michael Roukes, principal investigator of the paper and Caltech’s Frank J Roshek professor of physics, applied physics and bioengineering, was one of five scientists who, from 2011, worked alongside the White House Office of Science and Technology Policy to jump-start what became the Brain initiative.
Laurent Moreaux, Caltech senior research scientist and lead author on the Perspectives paper in Neuron Journal, explained: ‘We initiated the project to address the fact many major brain nuclei in mammals remain inaccessible to optical physiology because of the intrinsic depth limitation of free space optical microscopy, due to fundamental phenomenon of light scattering in opaque tissues.
‘To bypass this depth limitation, we envisioned using brain-implantable integrated photonics probes, similar in shape and size to the silicon shanks probe-based multi-electrode arrays, which would allow the ability to directly bring the microscope “into” the brain in a distributed fashion, and permit the use of functional imaging techniques to probe the activities of an unprecedented number of neurons at depth.’
Collaboration is key
The Caltech researchers say this new integrated neurophotonics method ‘has far greater potential than any current approach.’ It leverages recent advances in microchip-based integrated photonic and electronic circuitry, combines these with those from optogenetics and works by using minute assemblies of optical microchips that can be implanted inside the brain at any depth. These are used in combination with fluorescent molecular reporters and optogenetic actuators, to optically monitor neurons and control their activity, respectively.
The assemblies emit microscale beams of light to stimulate the genetically-modified neurons around them, and at the same time record the activity of these cells, revealing their function. Although the work is currently undertaken in animals, Roukes believes that it will ultimately be similarly used inside the human brain.
‘Dense recording at depth, that is the key,’ he said. ‘We will not be able to record all of the activity of the brain any time soon. But could we focus on some of its important computational structures in specific brain regions? That’s our motivation.’
Moreaux agrees, stating in the paper: ‘Our aim is not solely to identify ways to increase the total number of neurons that can be recorded from simultaneously,’ he said. ‘Instead, we explore the possibility of achieving dense recording from within a targeted tissue volume, to ultimately achieve complete interrogation of local brain circuit activity. We use the word interrogation to denote recording and direct causal manipulation of a brain circuit’s individual neurons by the application of patterned, deterministic stimulation with single-neuron resolution.’
Problem solving
The team believes that optogenetics can solve some of the problems related to neuroscience studies’ reliance on implanted electrodes to measure neurons’ electrical activity. This they say, on average, can reliably measure only a single neuron due to all the electrical activity in the brain. The brain does not use light to communicate, so optogenetics can make it easier to track large numbers of these signals.
However, many optogenetic brain studies are constrained by a significant physical limitation, explained Moreaux. ‘Brain tissue both scatters and absorbs light, which means that light shone in from outside the brain can travel only short distances within,’ he said. ‘Because of this, only regions less than about two millimetres from the brain’s surface can be examined optically. This is why the best-studied brain circuits are usually simple ones that relay sensory information, such as the sensory cortex in a mouse. They are located near the surface.’
Essentially, current optogenetics methods cannot easily offer information about circuits deeper in the brain. However, with this new integrated neurophotonics method, circuits buried deep in the brain could also benefit from valuable insight. The technique allows microscale elements of a complete imaging system to be implanted near complex neural circuits deeper in the brain, for example, the hippocampus region, associated with the formation of memory, and the striatum, which controls cognition.
‘This is one key advantage over an electrode-based approach,’ said Moreaux, ‘which does not really allow for much in terms of multiplicity per electrode, since recording electrical signals depends on the electrode being in very close proximity with the recorded cell.’
Roukes likened it to functional magnetic resonance imaging (fMRI), a similar technology which is currently used to image entire brains. ‘Each voxel, or three-dimension pixel, in an fMRI scan is typically about a cubic millimetre in volume and contains roughly 100,000 neurons,’ he said. ‘Each voxel, therefore, represents the average metabolic activity of all of these 100,000 cells. The overarching goal of integrated neurophotonics is to record what each neuron in that collection of 100,000 is doing in real time.’
High resolution
This means dense functional imaging of neuronal activity can now be used by the researchers in highly scattering neural tissue, providing cellular-scale resolution at arbitrary depths.
Moreaux said: ‘Our approach is based on implanting an entire lensless imaging system within the brain itself, by distributing dense arrays of microscale photonic emitter and detector pixels positioned on a 3D spatial lattice. These pixel arrays are integrated onto narrow silicon shanks, which leverage recent advances in silicon-nanoprobe-based fabrication. Used with functional molecular reporters and optogenetic actuators, this novel instrumentation offers the prospect of approaching the interrogation of all neuronal activity from within 100,000 neurons in a mouse cortex.’
The team’s long-term goal is to disseminate the advanced instrumentation of integrated neurophotonics to enable multi-institutional collaborations that will pioneer advanced neuroscience research with this novel technology. Previously, said Roukes, this type of neurotechnology development has relied mostly on research led by a single lab or investigator.
The team behind the Brain Initiative came together to bring the kind of large-scale partnerships to neuroscience research that have previously been seen in physical sciences. Now, Roukes says, integrated neurophotonics opens doors for such instrument-building teamwork. ‘Many of the building blocks have existed for a decade or more,’ he said. ‘But, until recently, there has just not been the vision, the will, and the funding available to put them all together to realise these powerful tools for neuroscience.’
For example, explained Moreaux, for the micro light beam delivery within the tissue from the neurophotonic probe’s light emitters, researchers had to promote a shift from the existing infrared photonics circuit paradigm, which is widely used in telecoms applications, to visible photonics circuits. ‘More specifically,’ he said, ‘blue light, to match the excitation optical spectrum of fluorescent biomarkers. We nucleated this effort at Caltech in our Kalvi
Nanoscience Institute (KNI) cleanroom and with our partners at Leti-CEA in France, and at AMF in Singapore. Of course the “reverse” efforts – developing optogenetic reporters that function in the infrared – also open up the possibility of using infrared photonics circuits as well. It is worth noting that the development of near-infrared optogenetic reporters is something that has been gaining momentum recently.’
Making a breakthrough
Some of these recent breakthroughs in molecular reporters can enable multimodal and multi-physical sensing. Likewise, optogenetic actuators enable optical control of neural activity, and the genetically encoded delivery of molecular reporters and actuators that provide specificity of cell type.
In terms of the challenges, in particular when it comes to the hardware, Moreaux feels that a key technological challenge is the integration of light delivery and photon sensors on the same chip with implantable form factors (shanks). ‘In other words,’ he said, ‘an integrated technology that allows for both the delivery of patterned, pulsed light and photon detection, such as integration of CMOS and photonics circuitry (silicone nitrate).’
The ability of the technology to be mass-produced will be essential if the technology is to be widely-adopted. So another challenge, said Moreaux, is that this integration capability must not only be achieved at a small, proof-of-concept scale, but also at the foundry scale. In addition, as integrated neurophotonics uses light to transduce the electrical signals of neuronal activity, development on the optical reporter side is also essential if the technology is to be used to map brain circuits and other applications.
‘While there have certainly been a lot of advancements in the area of optogenetic reporters in the past decade,’ said Moreaux, ‘there are certain challenges to consider in order to optimise their use in the context of integrated neurophotonics. For example, finer control of the spatial density of reporter expression: too dense, you lose the ability to separate between neurons, not dense enough, you lose out on taking full advantage of the technology.’
Beyond the technological challenges, of course, the monetary investment needed to develop this type of technology is also a huge hurdle. ‘Buy-in for this type of investment,’ said Moreaux ‘is more likely to gain traction in areas where there is a greater chance for larger tangible return on investment, such as in the clinical arena.’
Despite some of these challenges, Moreaux believes that the methodology is potentially scalable. ‘Multiple modules can be tiled to densely cover extended regions deep in the brain,’ he said. ‘We anticipate that this will ultimately permit interrogation – simultaneous recording and patterned stimulation of millions of neurons, at arbitrary positions and depths in the brain – to unveil dynamics of neural networks with single-cell resolution and specificity of cell type.’
Historical breakthroughs such as low-loss wafer-scale mass-production processes for visible-wavelength integrated nanophotonics have been achieved by working with foundry partners, said Moreaux. This, in turn, has allowed the demonstration of coherent optical beam formation in brain tissue; the realisation of beam-steerable phased arrays of micro-emitters and selective-plane illumination with micro-emitter probe arrays; and the creation of implantable, angle-selective single-photon microdetector arrays.
‘These constitute the fundamental building blocks for complete implantable visible-wavelength functional imaging systems with microscale dimensions,’ said Moreaux. ‘Further, we have validated the foundational physics for the integrated photonics paradigm by developing and implementing computational methods to evaluate the fluorescent photon yield in scattering media. With these tools, we have validated our computational approach for optical source separation and localisation and, thereby, have been able to identify practical, realisable architectures, that can enable dense volumetric activity reconstruction at depth.’
The systems are based on mass-production processes that are already routinely carried out in existing electronic- and photonic-chip foundries. Industry partnerships like these, believes Moreaux, will be essential in making this work a commercial reality. ‘Partnerships with mixed photonics and CMOS fabrication technology foundry partners will be important to co-integrate the different functional bricks of the technology on implantable shanks,’ he explained. ‘Likewise, industry partners with post-processing and optoelectronic packaging expertise. We have already been collaborating with CEA-Leti and AMF, as well as IBM and TSMC for the CMOS chip fabrication.’
All of these developments and partnerships together, believe the research team, validate the potential of integrated neurophotonics. This means, said Moreaux, that ‘they offer near-term prospects for wide deployment to the neuroscience research community.’
Iain Johnson of Lumencor explains Optogenetic techniques developed over the past 15 years, that have served neuroscientists well.
They provide spectrally and spatially resolved data of the functional complexity of neural networks, while avoiding direct physical interrogation using historically ubiquitous microelectrodes1. Optogenetics addresses cells and cellular function using non-destructive illumination, rather than invasive electrodes. ‘Genetics’ refers to transgenic expression of photoactivatable ion channel proteins, required to transduce light input into electrical activity in the cells of interest.
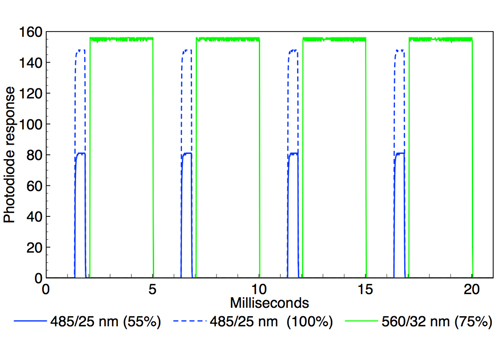
Figure 1: Oscilloscope recording of alternating 485nm (~0.5ms width) and 560nm (~3 ms width) output pulses generated by TTL triggering of a Spectra X light engine (Lumencor, USA). Two superimposed oscilloscope traces are shown in which the 485nm intensity is adjusted from 100 to 55 per cent via RS232 serial commands while the 560nm intensity remains constant. Temporal separation of the 485 and 560nm pulses is ~0.25ms.
Illumination sources used for optogenetic stimulation must meet certain requirements in terms of spectral, spatial and temporal output characteristics. This article considers these characteristics in relation to the performance of solid-state LED- and laser-based light engines.
The primary spectral output requirement is maximal intersection of the light engine spectral output with the action spectra of photoactivatable ion channel proteins. Currently, the most commonly used spectral outputs are 475nm for stimulation with channelrhodopsin (ChR2) and 575nm for inhibition with halorhodopsin (NpHR). LED-based light engines provide spectral outputs that are temporally discrete (figure 1) but spatially coincident. Additional spectral outputs facilitate use of novel photoproteins, and provide for excitation for fluorescent markers and voltage sensitive dyes, in parallel with optogenetic stimulation or inhibition.
For example, deeper tissue penetration can be attained by using near-infrared (>700nm) light for optogenetic stimulation2. It is common practice to use co-expression of yellow fluorescent protein (YFP) to enable localisation of ChR2 by fluorescence microscopy3. The choice of YFP is dictated by the fact that its fluorescence excitation (~510nm) is sufficiently wavelength-separated from photostimulation of ChR2 (~475nm) that the two processes can be independently initiated.
For in vitro experimentation on brain slice or cultured neuron preparations, LED-based light engines coupled to fluorescence microscopes provide widefield illumination suitable for simultaneous stimulation of thousands of neurons. For stimulation of ChR2, an irradiance threshold of ~1mW/mm2 at the sample plane is required, a level which is towards the lower end of the range used for conventional fluorescence microscopy. Spatially selective illumination can be generated by addition of a digital micromirror device (DMD)4.
For selective stimulation of individual cells, lasers that can be focused to diffraction limited spot sizes <10µm are required. In vivo experiments provide the added capacity to observe complex behavioural outcomes elicited by optogenetic stimulation. Light is typically delivered through a 200µm diameter multimode optical fibre, connected to a cannula implanted in the brain of an animal subject. Lasers provide better coupling efficiency into these optical fibres than LEDs, and are therefore the preferred light sources for in vivo optogenetics.
The electrical activity of neurons is modulated on the millisecond timescale. Therefore, illumination sources used for optogenetic stimulation must have the capacity for optical modulation on the same timescale (figure 1). A recent publication by Kubota and co-workers3 takes full advantage of the temporal and output power control provided by the Spectra X light engine (Lumencor, USA) to characterise photostimulation of ChR2-expressing rat dorsal root ganglion (DRG) neurons. Effects of varying light pulse durations (0.1 to 10ms) at constant intensity and varying stimulation intensities (2 to 78mW) at constant pulse width were recorded.
In addition, stimulation by pairs of 0.5ms pulses separated by varying intervals from 5 to 20ms was used to assess the effects of ChR2 desensitisation.
Quantitative comparisons of optogenetic and conventional electrical stimulation are included, providing a useful point of reference for readers previously unacquainted with the capabilities of optogenetic techniques. To find out more please visit https://lumencor.com
References
1 ES Boyden F1000 Biol Rep (2011) 3:11
2 S Chen, AZ Weitemier, TJ McHugh et al. Science (2018)
3 59:679–684 3 S Kubota, W Sidikejiang, K Seki et al. J Physiol (2019) 597:5025–5040
4 Zhu, O Fajardo, RW Friedrich et al. Nat Protoc (2012) 7:1410–1425