For anyone working in the medical technology space, bringing your revolutionary device from concept to market is an arduous process. Typically, a new product takes an average of three to seven years, which is not bad compared with the average 12 years for drugs, but woefully pedestrian when consumer products can appear on shelves in less than a year.
But the wait can be worth it. Photonics components are seen as key enablers of both current and next-generation medical devices, well-suited to non-invasive diagnosis, therapy and treatment, and solving a host of healthcare challenges society faces today. This is reflected in market statistics. According to Allied Market Research, the global biophotonics industry generated $52.17 billion in 2020, and is expected to balloon to $133.9 billion by 2030.
The EU is a leader in the global photonics market, second only to China, and regarded as a hub for innovation in medical photonics technologies. For instance, at the onset of the pandemic, European researchers in the CoNVat project rapidly developed an ultrasensitive photonic sensor to detect coronavirus and future pandemic viruses from a nasal swab or saliva. The sensor gave a reading in less than 15 minutes.

Sectioning of tissue samples from patients with head and neck cancer for validation of coherent Raman scattering microscopy at Jena University Hospital. Part of the Crimson project. Credit: Jena University Hospital
However, even in Europe, when a concept incorporates multiple high-precision complex photonics elements, patience is the watchword when it comes to bringing it to market. An example of this is the White Dwarf, a compact laser system for three-photon microscopy designed by Class 5 Photonics in Hamburg, Germany, and runner-up in this year’s Laser World of Photonics biophotonics and medical engineering Innovation Award. The device provides the highest peak power available for three-photon microscopy on the market, allowing neuroscientists to image deeper, faster and at better resolution.
From the first demonstration of the company’s optical parametric chirped pulse amplification technology in 2010 to the launch of the first White Dwarf system, it took six years. During a webcast subsequent to the awards ceremony, sales engineer Kolja Kolata explained the main challenges to bringing a product to market that meets industry expectations.
‘We are laser enthusiasts, all with a background in fundamental sciences, and we are driven by the clear vision to pioneer technology and push boundaries. But, as a self-funded company, it's important for us to look for trending markets and scalable products,’ he explained. ‘Proof-of-concept in the lab is easy – making a product out of this is always the biggest hurdle.’
Kolata said that the key for Class 5 Photonics was finding the right partners who could tell them what neuroscientists really required. ‘To have the reliability, to be in specs, to have a really robust process and in the end the most stable system, this is basically what was needed to push the boundaries of deep neural brain imaging and make it accessible to a broad community.’
At an earlier stage of development is Crimson, an ongoing three-and-a-half-year EU Horizon 2020 project, the objective of which is to drive technology developed in a previous five-year project called Vibra towards the market. ‘The technology measures the vibrations of molecules to perform remote, non-destructive chemical analysis that identifies different molecules with ultra-short light pulses,’ explained project coordinator Dario Polli of the Politecnico di Milano in Italy. ‘The aim is to see if we can understand the origin of diseases starting from the cellular level.’
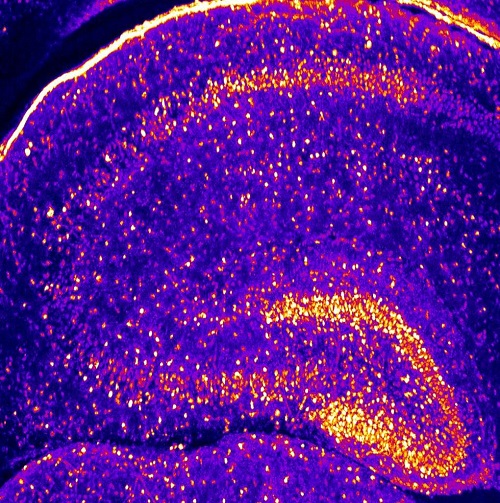
Two-photon image of a mouse brain slice with GFP labelled neurons. Captured by scientists at the research institute IF-CNRS as part of the Crimson project. Credit: IF-CNRS
To get there, the team will need to further develop new compact ultrafast lasers, label-free broadband coherent Raman scattering (CRS) detection schemes and advanced spectral analysis routines, and then bring them together into functional prototypes.
This work calls on the close collaboration of various different types of organisations dotted across Europe: ‘We have three universities who are really dealing with the core CRS technology itself, one company developing the laser specifically for this application, one company developing an innovative microscope, another company developing an endoscope, and a fourth company doing the data analysis,’ explained Polli. ‘And then we have three biomedical partners who will send us samples for testing the final prototype.’
By the end of the project in 2024, the technology should be capable of imaging samples ex vivo for mainstream biomedical research purposes. But the ultimate application is to probe inside the body, using an endoscope to touch and understand crucial properties of cells and tissues, including tumours, in near real-time. ‘You need to account for at least two and a half years more for a medical product,’ said Matteo Negro, CEO and CTO of project partner Cambridge Raman Imaging. ‘And that time frame is short because we have been investing in the medical design and development process from day one, ensuring it will be approved by the regulatory bodies and accepted by the clinical community – but it’s not a straightforward process.’

Wearable patch to monitor body signals, developed through the Medphab pilot line. Credit: Medphab and VTT
Jussi Hiltunen of the VTT Technical Research Centre of Finland recognises that bringing together disparate, often cutting-edge technologies into new medical photonics devices takes time, planning and the involvement of end-users, and that regulatory approval and community acceptance are central roadblocks in any devices intended for clinical application. But he also sees another problem. ‘Photonics is a fairly fragmented field of technologies, so it's quite complicated to establish the right production chains to make a functional system.’
In his role as coordinator of the Medphab project, Hiltunen aims to address these pressing issues. Also funded by Horizon 2020, Medphab is Europe’s first pilot line for photonics-based medical devices. Consisting of a consortium of 18 partners – including household names such as VTT and Philips, as well as research institutes and companies that have experience in ISO 13485 standardised manufacturing or strong expertise in photonics – the idea of the pilot line is to accelerate the commercialisation of photonics-based medical devices and reduce R&D cost. Often, this provides any company that has a good idea with the means and connections to bridge the ‘valley of death’ to low-volume production, but Medphab can help companies at any stage of the product development process. Hiltunen said that, in optimal cases, Medphab can reduce development time by up to 50 per cent.
‘The principle is that Medphab is an open access pilot line providing services to companies across the whole world,’ said Hiltunen. ‘What is exclusive to companies from EU countries – and other countries eligible under the Horizon 2020 programme, like Switzerland and the UK – is our Open Call programme that lasts until the end of next year, where we can provide up to 75 per cent financial support.
Lightcore’s role in the Crimson project is to develop a portable scanning coherent Raman scattering endoscopic imaging probe. Credit: Lightcore
Though he cannot talk about any of the projects Medphab is working on with external companies, the pilot line has been used by a selection of consortium members from the very start, to test and refine the Medphab operations and service model, and to provide use cases.
One such use case is Radisens Diagnostics’ Gemini technology. Gemini is a point-of-care in vitro diagnostics platform to quantify ferritin levels as a screen for iron deficiency. Iron deficiency is prevalent in blood donors, expectant and new mothers, and in children, adolescents and athletes. Quantitative diagnosis at the point of care – a feat currently not possible – would allow immediate clinical intervention.
The technology consists of an optical-based reader which automatically tests a latex-enhanced homogeneous ferritin immuno-assay on a single-use microfluidic cartridge. Medphab has been involved in the development of both the reader unit and the disposable microfluidic part. Radisens now has medical compatible demonstrators, equivalent to technology readiness level (TRL) 7 – TRL9 is when a product is market-ready.
Medphab is just one of many photonics pilot lines helping companies develop medical and other devices in the EU and beyond. Medphab and Crimson are also examples of successful public–private partnerships (PPPs) under the auspices of Photonics21, a European technology platform consisting of 3,000 members that coordinates photonics research and innovation priorities and brings together the whole economic value chain throughout Europe.
The Photonics21 PPP format has been a boon to European medical photonics innovators, from nanophotonic-based handheld point-of-care analysis devices for early diagnosis of cancer to compact photoacoustic imaging systems for screening, diagnosis and monitoring of cardiovascular disease. Currently, Photonics21 has 17 PPPs on its roster developing photonics technologies for health applications. And this number can only rise – according to Photonics21, the photonics SME industry is committed to investing €100 billion in research and innovation in Europe between 2021 and 2027. During this time, the European Commission will invest at least €480 million as part of Horizon 2020’s successor, Horizon Europe. All of this investment in a healthy, well-organised photonics ecosystem means we can expect a lot more new and innovative medical photonics devices being wielded by researchers and clinicians soon.
As the world moves from standardised medicine to greater personalisation, and from outmoded methods for responding to public health crises to faster, more cohesive protocols, the need for non-invasive technologies to diagnose, monitor and treat medical conditions continues to grow. Spectral technologies are helping medical researchers and device developers to meet those demands with solutions that are simpler, smarter and more robust than ever.
Background
When the miniaturisation of spectral technologies began in the late 1990s, those early instruments benefited from a perfect storm of technological circumstances: the development of imaging detectors for mass-volume markets, which lowered system costs and allowed designers to shrink the instrument footprint; and the evolution of personal computers, which enabled spectrometers to process high-speed spectral data and the growth of fibre optics, which made it easier to bring the spectrometer to the sample.
These developments have benefited scientific, research and industrial sectors and spurred additional advances in spectrometer development. Successive generations of miniature spectrometers have become faster, more accurate and more compatible with data transmission communication protocols, including ethernet and wi-fi. This has virtually eliminated performance trade-offs inherent to earlier miniature spectrometers and makes them more scalable for OEMs and other high-volume users.
In biomedical and life science applications, spectrometers are routinely integrated into other devices, combined with fibre optic components for use as subassemblies, or designed into fully realised instruments for applications ranging from biofluidics control to molecular diagnostics. Below are two more examples.
Quality control of catheters
A leading medical device manufacturer recently assessed the use of spectroscopy as part of its quality control for antimicrobial-impregnated catheters. To help prevent biofilm formation and subsequent infection, the company coats its implantable catheter tubes with a polymer solution containing a suspended antimicrobial agent. This coating is then cured.
To ensure that minimum antimicrobial concentrations are present and the coating is applied uniformly, a liquefied sample of the cured antimicrobial layer is sent to an outside lab for high-performance liquid chromatography (HPLC) quantification. This process can be time-consuming, expensive and destructive to the product and does not guarantee a uniform layer. The manufacturer needed an on-site system that could measure the concentration and coating uniformity quickly, accurately and with no sample destruction.
To measure antimicrobial concentrations, a high-sensitivity miniature spectrometer was combined with a high-power tungsten halogen lamp and reflection probe mounted in a ring stand for repeatability. The average of each concentration spectra was taken and then divided by a clear reference sample spectrum. This resulted in the average relative reflectance of each concentration (Figure 1). These values plotted against the HPLC concentration data revealed an R2 correlation value of 0.9924. The striking correlation between relative reflectance of a miniature UV-visible spectrometer and the HPLC-determined concentrations empowered the manufacturer to implement this set-up into its quality control process.
Figure 1: An Ocean Insight miniature spectrometer (350-925nm) measures the reflection of coatings used on antimicrobial-impregnated catheters. Repeatable coating concentration and uniformity results ensure catheter integrity
Quantifying protein concentration
Biological tests and processes have seen a recent burst in attention from new technologies hitting the mainstream, such as rapid virus detection, custom-engineered medical treatments and at-home genetic tests. In many of these tests, UV absorbance is used to determine the concentration of a constituent.
Depending on the sample and application, UV protein detection may be necessary at very low limits, or at extremely thick concentrations. The power to perform accurate measurements at both extremes is available via high-sensitivity miniature spectrometers, which provide excellent resolution at normal light levels, as well as stable, meaningful readings at the lowest intensities.
As testing demonstrates, a miniature spectrometer can successfully generate a standard curve for bovine serum albumin (BSA). BSA is a protein derived from cows that is frequently used for biochemical applications.
Why is this important? The generation of a standard curve (also known as a calibration curve), is a typical procedure in many biomedical, diagnostic and life science research laboratories. An accurate determination of protein concentration is the first step in assays involving proteins. With high-performance, newer-model miniature spectrometers, researchers and developers have a flexible set of tools for UV absorbance measurements, including those required for determining the concentration of an unknown protein.
Summary
Pushing the frontiers of what’s possible in understanding disease, developing diagnostics and using biotechnology to advance therapies demands a versatile toolkit of analytical instruments and the guidance of knowledgeable partners.
Today’s miniature modular spectrometers, both customisable and flexible, are ideal for supporting the application of spectroscopy techniques to biomedical research and diagnostic device testing and development.
Further information www.oceaninsight.com