Doctors’ trust in diagnostic testing has decreased during the pandemic due to exaggerated and inaccurate claims made by test manufacturers, a panel of doctors said during a Photonics West discussion.
Despite the huge potential for optics and photonics technologies to fill gaps within point-of-care diagnostics and therapeutics, trust between manufacturers and medical professionals needs to be re-built, the panel said.
This, in part, will involve rethinking the funding landscape for diagnostic device development, which currently deprioritises large-scale clinical trials, leading to tests that underperform in larger sample sizes.
The comments were part of the Covid-19: perspectives from low-resource settings talk at the Photonics West virtual conference in March.
Mistrust in tests
Professor Kev Dhaliwal, consultant physician in respiratory medicine and personal chair of molecular imaging and healthcare at the University of Edinburgh, redirected his research to study the disease progression of Covid-19 when the pandemic hit. His group in Edinburgh investigates disruptive optical technologies to advance respiratory medicine and critical care.
‘We evaluated a whole range of optical diagnostic technologies, from saliva- to blood-based, and found real challenges in terms of performance.
‘We were finding that huge claims were being made about diagnostics,’ he said.
Omai Garner, director of clinical microbiology at the UCLA Health System hospital, agreed: ‘There has been a lot of misinformation out there.’
The Health System’s 120-person lab carries out infectious disease diagnosis in Los Angeles. At the start of the pandemic it quickly pivoted to perform real-time PCR testing.
‘Unfortunately, there has been a lot of poor tests, of which the marketing got exceptionally good media coverage – even globally – and they’ve been widespread for profit,’ he said.
‘[Covid diagnostics] has been an unfortunately lucrative space – both in the private industry and from a research perspective,’ Garner continued. ‘So, everybody with an idea is getting on board. And because it has been a little less regulated than it should, there have been products that have made it to market, distributed in the US and globally, that have just been garbage tests,’ Garner continued.
Clinical decisions are then made on the results of these tests, Garner added, with both false negative and positive tests potentially leading to increased spread of the virus.
The existence of poor tests is a result of not enough robust analysis of the techniques created. ‘A lot of tests have looked really good on a few hundred patients. You take that data and can deploy and roll it out. But then with 1,000 or a few thousand [patients] the test completely falls apart,’ said Garner.
This is related to how newly-developed techniques and devices are funded, Garner highlighted.
‘It’s relatively easy to get the funding to do the research to attempt to create a new diagnostic. But it is incredibly difficult to get funding to do the clinical trials of that diagnostic in an under-served space. I’m going to say it’s almost impossible,’ he said. ‘All of the major funding organisations have this particular issue.
‘The group that wants to fund you to make the device, is actually a separate group that wants to fund you to prove you can use this device effectively in an under-served area.
‘There is a huge gap in the point-of-care world, in making something analytically sensitive and making something that’s clinically useful in an under-served area. That gap is poorly studied. You don’t end up making a device, you end up publishing a lot of papers on a new point-of-care technique that has this beautiful sensitivity, and then it just ends.
‘Covid-19 and other infectious diseases point out this gap that needs to be addressed, from a funding perspective, if we are ever going to make effective, sensitive, cheap diagnostics that can be used globally,’ Garner said.
Beyond PCR: huge opportunities for optical tests
In addition to challenges associated with the performance, relying mainly on one type of diagnostic test makes you vulnerable to supply issues, the panellists said, particularly with techniques that require several components.
‘We desperately need to develop new diagnostic methods that might replace PCR, to deal with future pandemics,’ said Dr Karin Nielson, a professor of paediatrics in the infectious diseases division at UCLA Children’s Hospital. Her team had been researching the neurodevelopment of children affected by the Zika virus in Brazil before the pandemic. They then switched to studying Covid-19 transmission between mothers and babies, and its effect on children over time.
‘Not having diagnostic ability severely prevented us from taking preventative measures. I do not trust the current numbers – they are clearly an under-representation,’ she said.
‘Everything is outsourced. It has negatively impacted the pandemic and undermined everyone’s trust.’
UCLA Health’s Garner added that the ideal test would be a point-of-care device, in which only the target molecule needs to be changed. ‘But for that you need to sort out the funding issues,’ he said.
In addition to diagnostics, photonics has a huge potential in therapeutic development, which, until now, has been based on a largely trial-and-error approach.
The University of Edinburgh’s Dhaliwal said doctors desperately need better tools to help them understand how infectious diseases progress inside the human body, for example with more advanced respiratory endoscopes. ‘We had to move to post-mortems to understand the lung. Better experimental interventional medicine is needed – getting photonics deep inside humans, where we can be at the site of disease activity, understand the players, deliver drugs locally, look at the local effect of photonic biomarkers.
‘We’ve been trying to treat [Covid-19] as a black box. The trials try drug a, drug b, drug c and then just hope for results. If we could characterise the disease in vivo in humans, there will be jumps that occur. That is the same for all diseases, but we have really learnt now with pulmonary medicine, lung injury, infection inflammation, it is a black box,’ he said.

More advanced endoscopes are key for understanding better how diseases affect the lungs
Dhaliwal referred to acute respiratory distress syndrome (ARDS) that has been studied for the last 20 years, with minimal advances in understanding and treating the disease. ‘Trials have failed for ARDS. A lot of drug companies were not interested in acute infection or acute pathogenicity.
Photonics can make a huge difference to changing that paradigm. We need to take photonics into the person,’ he said.
As vaccinations are rolled out, Nielson said antibody testing will become more important, needing to be simple, low-cost and reliable. ‘Photonics could help with this development of rapid diagnostics for the assessment of antibodies,’ she said.
This will also be crucial in low-resource areas, where vaccines will be in limited supply, added Dr Neema Kaseje, of Doctors Without Borders, who has helped develop Covid-19 health strategies in Kenya.
‘In Kenya we have received one million doses of the vaccine and the population is 55 million. We will never receive 55 million doses, but if there is antibody testing, we could manage our resources more effectively, saving the vaccines for those with no antibodies,’ she said.
It comes down to trust
For new technologies to be translated into clinics successfully, funding for diagnostics needs to be re-thought, Garner reiterated, which is key for end-users to gain trust in new technologies.
‘There is funding to make that test, to do the first 100 patients, but there is no funding to do a large-scale POC clinical trial,’ he said. ‘Until that issue is addressed, it’s very hard for clinicians to then look at what you have with new technology, and trust that result. I run a gold-standard diagnostic laboratory and it is sometimes hard to trust the results coming out there. Because they just do not match what is going on diagnostically,’ Garner continued.
‘If you have a test that goes wrong once, you’re already starting to lose the trust of the clinician, if you have a test that goes wrong 50 per cent of the time, that test is actually unusable.
‘If the funding is there to really study the real-world application of these diagnostics, and to study it quickly – in a setting where you have a robust dataset – that is how we build trust,’ he said. ‘But it takes a change from the organisations that are providing that funding.’
Optical modelling of biomedical applications using TracePro
Optical-based biomedical systems can span a wide range of applications from wearables, pulse oximeters, absorption spectroscopy, fluorescence microscopy and many others. These systems use light sources of varying types and wavelength ranges, including UV, visible, and infrared. Optical design and analysis programs, such as TracePro, allow designers and engineers to design, analyse and optimise these types of systems in a virtual environment. This allows for easy and efficient modification of the system parameters, and helps to speed the design process and reduce development costs.
TracePro allows for an easy file exchange with different CAD programs. Designers and engineers can use their preferred CAD program for the mechanical design, then export the model to TracePro for the optical analysis.
Typical file formats are SAT, STEP and IGES. TracePro is based on a 3D solid modelling CAD kernel, so users can also use TracePro for their CAD design.
TracePro features an interactive optimiser that gives engineers and designers the capability of optimising the design of light guides, lenses, reflectors, or almost any other optical element automatically to meet a user-defined goal such as flux, illumination pattern or uniformity.
The saying 'garbage in equals garbage out' can be applied to many things, including optical analysis software. To get the best results from an optical analysis, it is necessary to have accurate properties. This can include material properties, surface properties, scattering properties, as well as light source properties. TracePro includes a built-in catalogue on many optical properties, as well as the ability for users to easily add their own properties to the database at any time.
The modelling of the light sources is a critical part of the process.
Being able to model the spatial, angular and spectral distribution of the source is extremely important.
Some examples of source modelling tools are grid sources, surface source properties and rayfiles. Rayfiles can be downloaded from many LED manufacturer’s websites. TracePro can use all of these types of light source models. Light source models can also be mixed and matched.
Once a raytrace has been run, the results need to be analysed. Analysis can include illumination patterns, flux on a detector, angular/intensity distributions, luminance and radiance, polarisation plots, 3D irradiance/illuminance, photorealistic rendering, optical path length, and time-of-flight plots. Results may also need to be displayed in either radiometric or photometric units. TracePro gives users the ability to show all of these results and more.
The design and analysis of biomedical applications often requires specialised tools and capabilities in the optical analysis software. These can include fluorescence, birefringence, bulk or internal scattering, and importance sampling. TracePro includes all of these tools. Accurate modelling and analysis of biomedical systems without these capabilities can be difficult, if not impossible.
The following examples will illustrate some of these capabilities in TracePro. The first example is a fluorescence microplate reader. This model was made using TracePro, Oslo, and RayViz for Solidworks. This method allowed each program to be used for what it does best. Oslo was used to design and optimise the lenses, RayViz for Solidworks was used for the mechanical design, and then the system was imported into TracePro for a full optical analysis.
In this example an excitation source is used to illuminate a sample in a sample holder. A fluorescence property is applied to the sample. This sample will then fluoresce according to the fluorescent property applied. An important note is that the fluorescence emission will be from a 3D volume and not just from a surface. Figure 1 shows the excitation and emission beams.
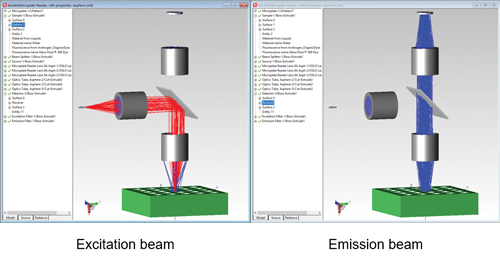
Figure 1: Fluorescence microplate reader excitation and emission beams. A red LED shines through a model of a human finger. The finger model includes skin, bone, blood vessels and tendons. A detector on the opposite side of the finger from the source detects light that has passed through the finger. A major factor in this type of device is accurately modelling the scattering of the skin, bone, blood vessels and tendons. TracePro’s Bulk Scatter property and included catalogue of tissue properties makes this a straightforward process.
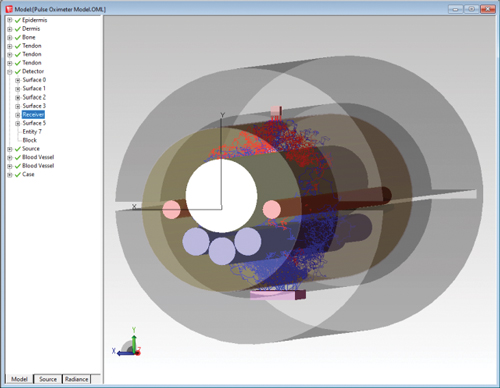
Figure 2: Pulse oximeter example shows the path of rays getting to the detector through a finger model. Designing, modelling and analysing biomedical systems requires an optical design program that combines excellent CAD tools, accurate properties, accurate source modelling and tools to analyse results. Special features such as fluorescence and bulk scattering are often also required. TracePro provides all these tools and more.


