Testing for Covid-19 has been a critical part of every country’s response to the pandemic. Back on the 16 March, the World Health Organisation’s head, Tedros Adhanom Ghebreyesus was telling countries ‘test, test, test’; South Korea, in particular, has been praised for its approach to controlling the spread of the virus through extensive testing early on when its outbreak first occurred.
One of the most common methods for testing whether a person has Covid-19 while they are displaying symptoms – as opposed to testing for antibodies to say if a person has had the virus and recovered – is using a technique called polymerase chain reaction (PCR). This relies on detecting fluorescence from tagged sections of amplified DNA, which in turn relies on the optical filters and optical coatings employed in these diagnostic kits.
Edmund Optics’ product marketing manager, Daniel Adams, explained that in a typical fluorescence system, bandpass filters are used to select both the light used to excite the sample and the light emitted after excitation.
‘To produce a clear signal, it is critical that these filters transmit as much light in the passband and block as much light as possible outside it,’ he said. ‘It is also important that the filter curve transitions quickly from transmission to blocking to minimise the amount of unwanted light incident on the detector.’
Edmund Optics’ optical components have been used in a diagnostic device from South Korean firm Mico Biomed. The Veri-Q PCR 316 can detect Covid-19 within one hour based on reverse transcription polymerase chain reaction (rRT-PCR). The device uses optical filters, beamsplitters, lenses, and mirrors to manipulate the excitation light and focus the emitted signal onto a detector.
The Danish firm Qlife has licenced a test for Covid-19 in its portable diagnostic kit called Egoo. The technology is currently being validated as a Research Use Only product, but the company plans to obtain a CE mark to sell it commercially by autumn 2020.
Egoo uses an RNA amplification method that differs from PCR, but still relies on fluorescence filters, this time provided by Delta Optical Thin Film.
Delta’s optical filters for point of care instruments, such as Egoo, are designed to provide excellent optical performance in a small size. Shrinking the filter has its own challenges, not least because the filters used in small, portable equipment often have to accommodate wide angles of incidence in order to collect enough light. As the angle of incidence increases it can change the performance of the filter, as the centre or edge wavelengths can shift and the light can split into different polarised states. These unwanted effects are minimised by the design and production techniques, including coating methods.

Delta is working with two companies developing rapid Covid-19 detection tests which include its fluorescence filters
Thin film coatings are essentially what give a lot of the properties of the filter, the high transmission in the desired wavelengths and the steep edges into the wavelengths that are blocked. The advances in coating deposition methods over the last 20 or more years have been one of the big enablers to making high performance filters. The range of deposition methods vary, and companies like Laser Components or Schott will offer different coating options depending on the desired optical performance. Laser Components, for instance, has access to e-beam, ion-assisted deposition, and ion beam sputtering machines.
For a portable device like Egoo, the optical filters inside have to remain stable as temperature and humidity fluctuates, which typically requires a hard coating. Delta Optical Thin Film’s ultra-hard coating method is based on a plasma deposition process that packs the coating layers very tightly on top of one another. Water uptake in these coatings is therefore minimal. Delta also says it can dice filters into small sizes, which is important for portable point of care devices.
Moth-eye structures
Moving away from point of care instruments, Edmund Optics has recently released optical components with a new type of anti-reflective coating. Instead of traditional dielectric films, the firm’s Nebular coating technology uses sub-wavelength nano-structures on the optical surface to provide high transmission and high laser damage thresholds.
The Nebular nano-structured anti-reflective surfaces are designed to meet the requirements of demanding laser applications that need high transmission and high damage thresholds. The surface structure, which consists of an array of bumps with a spacing between them less than the wavelength of visible light, is produced by an etching method. The texturing, also known as the moth-eye effect, acts as a refractive index gradient, as opposed to a thin film coating which gets its properties from the interference of light between layers.
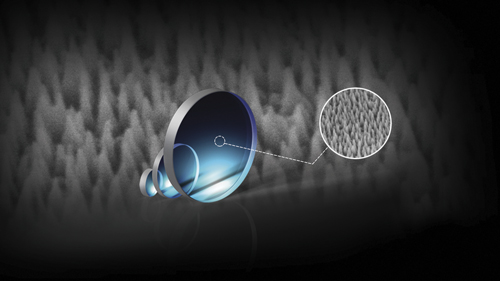
Edmund Optics has released laser line windows processed with its Nebular surface structuring method
‘Traditional thin film coatings have drawbacks when used with high energy laser sources,’ explained Adams. ‘They are prone to imperfections which can cause diffraction, absorption or scattering and this often leads to laser induced damage.’
In addition to solving these issues when used in high-energy laser sources, the Nebular coatings are also able to perform over a much broader range of wavelengths and angles of incidence than traditional thin films, according to Adams.
It’s the high laser damage threshold that is, at the moment, what this surface structuring method is being used to create. But the other properties of these alternative anti-reflective coatings – the broad range of wavelengths and angles of incidence – are benefits that will undoubtedly be explored. Nano-structured surfaces might not find their way into optical tests for Covid-19, but with commercial optics now being textured in this way, other uses of this technology are sure to follow.
The role of thin film optics in qPCR and Covid-19 detection
Filter features such as high transmission, steep edges, low ripple and deep blocking are important when testing many samples, says Dr Rance Fortenberry, director of technology at Alluxa
The current Covid-19 pandemic has highlighted the need for rapid and accurate quantitative analysis of dangerous pathogens, particularly Sars-Cov-2. Fortunately, our ability to determine the structure of new and dangerous viruses has continued to improve since the invention of polymerase chain reaction (PCR), which enables the production of billions of copies of a single DNA sample.
The ‘gold standard’ for DNA detection today is quantitative polymerase chain reaction (qPCR), which combines standard PCR with dye molecules whose fluorescent output increases as the reaction proceeds. This optical monitoring of the PCR reaction in real time – in minutes rather than days – has revolutionised PCR based detection of DNA.
qPCR is based on a multistep thermal cycle (Figure 1). Each cycle, which can take less than a minute, results in a doubling of DNA. Step one separates the sample DNA into single strands by raising the temperature to >90°C, which breaks the hydrogen bonds holding the double stranded DNA together. Step two is an annealing step where the temperature is lowered (55 to 60°C) so that short DNA sequences called primers and probes can attach to the single-stranded DNA. Step three is an extension step where the polymerase enzyme builds the two single strands of DNA into two double strands. Note that although the Sars-Cov-2 virus is RNA based, reverse transcription is used to convert the RNA to complementary DNA before qPCR testing begins.
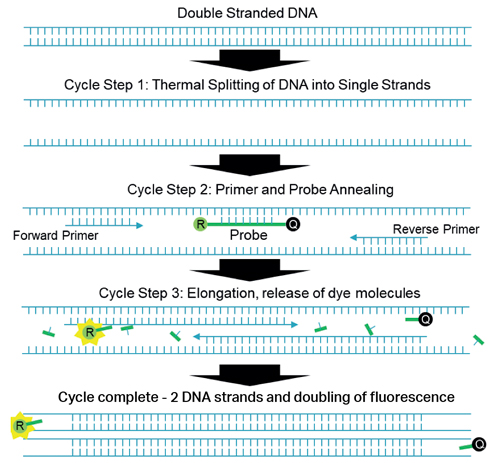
Figure 1: One cycle of qPCR process showing a doubling of DNA and fluorescence
The short probe and primer DNA sequences are designed to match specific sections of the DNA under analysis, and the probe has the fluorescent dye, or reporter, molecule directly attached along with a quencher molecule. The presence of the quencher molecule prevents fluorescence by allowing only non-radiative energy transfer via a mechanism called Förster resonance energy transfer (FRET). During the extension step the polymerase enzyme breaks up the probe DNA, separates the molecules and removes the quenching effect, leaving the reporter molecule free to fluoresce. Since one reporter molecule is released for each double stranded DNA molecule created, the optical signal scales linearly with the amount of DNA created, or exponentially with cycle number or time.
Typically, multiple sets of primers and probes are created to detect different regions in Sars-Cov-2. Like other coronaviruses, the Sars-Cov-2 virus has four structural proteins known as the S (spike), E (envelope), M (membrane), and N (nucleocapsid). The first three make up the viral envelope surrounding the last, which holds the RNA genome. The United States CDC standard assay uses three N-gene probes, two (N1 & N2) specific to Sars-Cov-2, and one (N3) common to all coronaviruses. A fourth primer and probe set are used to detect human RNAse P (RP) as a check on specimen quality, along with positive and negative controls that check for errors or contamination.
The US CDC assays employ carboxyfluorescein (FAM) as the reporter molecule for all probes, with absorption and emission wavelengths at 495nm and 520nm. In testing, the different probes are in separate wells within a standard 96-well assay plate, with some wells containing probes for N1, others N2, N3, or RP, and others containing positive and negative controls.
When using only a single dye – FAM is the nearly universal choice due to its high brightness – the optics required is particularly simple. A light source (LED, laser, or filtered white light) excites all wells of the assay plate simultaneously with light in the FAM absorption band, and the output fluorescence is passed through a thin film bandpass filter centered on the FAM emission wavelength. The filter output is then imaged onto a CCD camera that quantifies the amount of light emitted by each well as a function of time or cycle number.
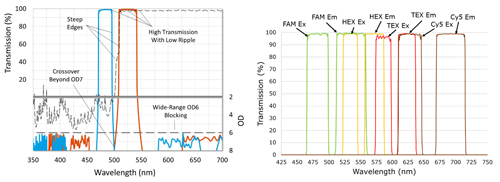
Figure 2: a) Important optical filter properties. b) Four channel setup showing excitation and emission filters multiplexing FAM, HEX, TEX, and Cy5 dyes for Sars-Cov-2 detection
When many samples are to be measured, this relatively simple approach can be improved by using probes with dyes that fluoresce at different wavelengths. Multiplexing more than one probe in a single well can significantly increase the number of samples that can be tested within a given time or budget. However, it is important dye combinations are selected with excitation and emission wavelengths that have minimal overlap, to reduce optical crosstalk, along with quality optical components.
In a single optical channel, higher filter blocking generally reduces unwanted background signal and results in a lower threshold of detection, but in multiplexing systems, optical crosstalk is more critical and can significantly limit testing ability. With available hardware platforms capable of handling four optical channels available today, and new qPCR platforms that multiplex five optical channels starting to arrive, optical filter features such as high transmission, steep edges, low ripple and deep blocking become more important.


