Characterising the performance of advanced optical microscopes – which provide high-resolution views of the structure and function of cells and molecular compounds – is essential for their optimisation and quality control. This is especially true during longitudinal experiments, in which information on a particular individual or group is gathered over extended periods of time.
According to researchers at the University of Illinois Urbana-Champaign, recent advances in nonlinear optical imaging microscopy techniques – those which rely on nonlinear interactions between light and matter to generate images – have increased the adoption of imaging modalities that rely on high-intensity illumination. The scientists say that calibrating the performance of these imaging systems poses a new set of requirements on the calibration samples, called phantoms, used to assess their imaging performance. However, creating reliable phantoms has previously proven challenging. Phantoms based on fluorescent beads have typically been relied on to provide rapid insight into the imaging performance of microscopes.
However, the fluorescent dyes in such beads decay rapidly due to a process known as photobleaching, leading them to become dimmer over time. To partially counteract this, fresh imaging samples need to be continuously prepared, which is not only time-consuming, but also requires care to be taken to avoid imaging the same beads for too long before they bleach.
There is therefore a need for a convenient and ‘shelf-stable’ phantom alternative to fluorescent beads, according to the researchers. These microscopy calibration Diamonds are forever targets would need to be resistant to bleaching, while also being able to provide a contrast across the multiple non-linear imaging modalities in use.
Nanodiamond-based phantoms
In collaboration with industry partners, the researchers have consequently turned to microscopic nanodiamonds – durable microscopic particles with small amounts of other chemical elements trapped inside as impurities – to develop a stable fluorescent phantom alternative.
The work, published in Photonics Research, promises wide-reaching applicability for microscopy research and quality control alike. It establishes fluorescent nanodiamonds’ efficacy for producing stable microscopic images.
‘[They] are unique in the way that they do not bleach,’ said Mantas Žurauskas, an imaging research scientist at the University of Illinois Urbana-Champaign’s Beckman Institute for Advanced Science and Technology, who led the research. ‘Each time you look at them, they look the same. That’s very rare in fluorescence microscopy.’
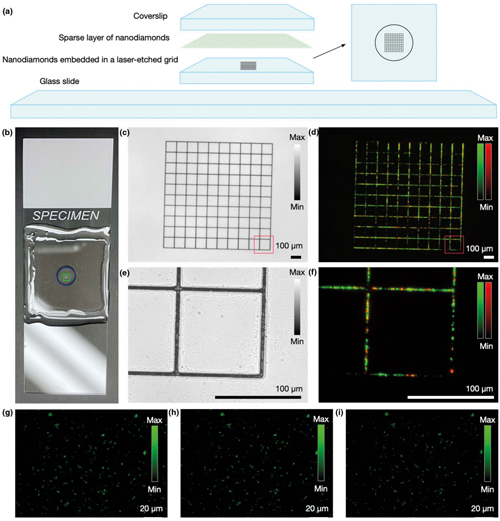
(a) Structure of a nanodiamond-based imaging target. (b) Photograph of an assembled target. (c) Laser-machined structure is visible in transmission widefield imaging mode. (d) Nanodiamonds embedded in the grid facilitate view finding in fluorescence imaging. (e) + (f) Magnified images of a portion of the grid. (g)–(i) Images of the layer with sparse randomly distributed 100nm nanodiamond particles, captured (g) immediately after assembling the phantom, (h) after one month of storage, and (i) after six months of storage. The measured fluorescence intensity for individual particles varied by less than 2 per cent (Beckman Institute, University of Illinois Urbana-Champaign)
Fluorescent nanodiamonds’ stability and longevity therefore allows their continuous reuse as a calibration tool, eliminating the labour-intensive preparation researchers are typically required to undergo. Their fluorescence is emitted by the defects in the lattice, with no signs of bleaching being shown over a period of several hours.
The researchers describe the nanodiamonds as a ‘first-aid kit for a microscope’.
‘There is potential that this is going to become a standard calibration tool in fluorescence microscopy worldwide,’ remarked Žurauskas. ‘This sample is so convenient and so easy to use that it is hopefully going to make a large impact.’ In their paper, the researchers describe the new phantom design as comprising a pair of two-dimensional nanodiamond layers supported by a glass structure. For the first layer, single nanodiamond particles are dispersed sparsely and randomly which, according to the researchers, enables imaging quality characterisation.
‘A single image of a dense layer of randomly distributed nanodiamonds, positioned at the focal plane of a microscope, can provide information for rapid assessment of system performance,’ they explain in their paper. ‘The optical quality of the imaging system and the presence of aberrations due to a system drift over time can be easily detected by assessing changes in the spatial frequency spectrum while imaging the same location of the dense lawn of randomly distributed subresolution nanodiamonds. Also, the parameters such as the uniformity of the fluorescence intensity across a field of view can reveal vignetting and imaging plane curvature. Lastly, the average intensity value from a fraction of the top intensity values could offer insights about signal excitation efficiency, while the low baseline values could indicate background noise from environmental illumination in the system.’
The second adjacent layer has nanodiamonds arranged in a grid pattern, which is used for calibrating x and y scale dimensions and facilitates finding the same area during repeated imaging events – described by Žurauskas as a ‘viewfinder’ grid. It can also serve as a microscopic ruler for measuring the size of the area being imaged. The layer consists of a glass coverslip with a laser-etched 1 x 1mm grid subdivided into 100μm squares. The grooves in the laser-etched grid are 1μm deep and are readily visible in transmission microscopy and in a third-harmonic generation (THG) imaging channel through inverse contrast due to a THG signal from a glass-air interface.
Fluorescent nanodiamonds were embedded in the etched grooves of the grid to further enhance the visibility of the grid in other channels, and particularly in fluorescence imaging channels. The particles were inserted into the laser-etched grooves through dry deposition of the nanoparticle layer, and by subsequent drycleaning of the glass substrate to remove the particles present on the raised flat areas and not embedded within the grooves of the grid.
Suited for a wide range of microscopy systems
The researchers anticipate that their nanodiamond-based phantoms will be valuable for optical instrumentation developers and for imaging facilities to rapidly assess the imaging performance of microscopes.
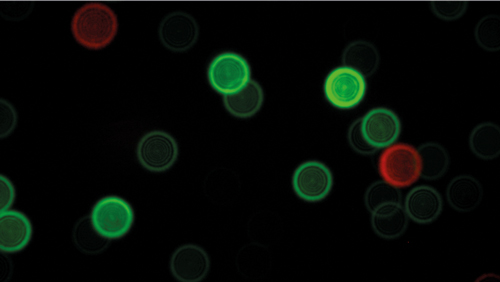
A microscopy image of fluorescent nanodiamonds (Beckman Institute, University of Illinois Urbana-Champaign)
‘Additionally, we expect that for microscope users, an image of our nanodiamond-based phantoms taken before each imaging session can provide for quality control of system performance during longitudinal or comparative studies, or for image normalisation during image data analysis,’ Žurauskas added. The researchers note in their paper that their new phantoms are also suited to adaptive optics-based microscopy systems that rely on image quality metrics for estimating the optical wavefront. The stable fluorescence signal, coupled with good spatial frequency coverage in the object plane, will improve the accuracy of iterative measurements when calibrating the adaptive element and measuring the system-induced aberrations.
They also highlight that fluorescent nanodiamonds will be particularly suited to stimulated emission depletion (STED) and stochastic optical reconstruction microscopy (STORM) super-resolution microscopy techniques, as they are especially sensitive to photobleaching. Therefore, the scientists anticipate that nanodiamond-based imaging phantoms could be integrated into system development and maintenance pipelines for these systems.
In the paper, the researchers have explored nanodiamonds containing two types of colour centres, nitrogen-vacancy (NV, with emission centred around 680nm) and nitrogen-vacancy-nitrogen (NVN, with emission centered around 580nm). Other colour centers are also actively being developed. Specifically, nanodiamonds containing N3 are emerging as a commercially available product that emit fluorescence centered at 450nm, providing coverage for the blue end of the spectrum. ‘The improved availability of alternative colour centres will allow for good coverage across the visible optical spectrum,’ the researchers remarked.
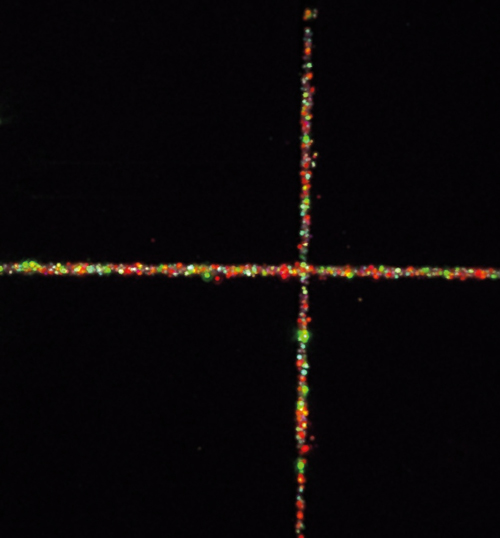
A microscopy image of the viewfinder grid with embedded nanodiamonds
Industry partners
The researchers currently have industry partners evaluating their nanodiamond-based imaging phantom for wider use. ‘One company is LiveBx, a small spinoff of the university,’ said Žurauskas. ‘That particular company is interested in how these phantoms can be used to improve their system.’ Meanwhile, industry partner GlaxoSmithKline is also working to assess the new phantom for quality control applications in its own biomedical research labs.
Overall, the technology represents an important scientific advancement for calibrating microscopy systems and the images they generate, and points toward future research in creating more advanced and stable phantoms.
‘Nanodiamonds have near-infinite photostability and can serve as a contrast agent for a wide variety of optical microscopy system types,’ concluded Žurauskas. ‘Because of these properties, our maging phantom provides a stable, low-maintenance, and easy-to use alternative to other imaging system characterisation methods. As a result, we anticipate that nanodiamond-based imaging targets and phantoms will have the potential to be widely adopted in microscopy laboratories and imaging facilities as a standard calibration and characterisation tool.’
Reference Paper in Photonics Research: https://doi.org/10.1364/PRJ.434236
Laser light engines for advanced fluorescence imaging
Increasing spatial resolution in fluorescence microscopy generally involves spatial constraints on the illumination field. In so doing, losses in the microscopy light budget are incurred, which must be compensated for with increased output from the light source. For example, super-resolution microscopy by single-molecule detection localisation is capable of a 10-fold increase in spatial resolution compared with widefield fluorescence microscopy; however, it requires irradiance (power/unit area) on the order of 104 mW/mm2 .

Figure 1. A Celesta Light Engine, overall dimensions and light output port
Lumencor’s CELESTA and ZIVA Light Engines are designed to meet and exceed even these very demanding illumination requirements. Both house an array of up to eight independently operable solid-state lasers within an optics and electronics control framework. Their many capabilities are assembled in a compact, turnkey, bench-top device (figure 1). Laser outputs are spatially coincident, so the illumination pattern does not change when output is switched between laser lines (figure 2). This is a critical characteristic in multicolor fluorescence imaging using sequential illumination from two, three or more laser colors.
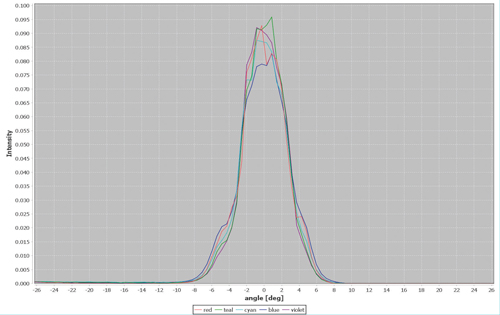
Figure 2. Angular distribution of the 405 nm (Violet), 446 nm (Blue), 477 nm (Cyan), 518 nm (Teal) and 637 nm (Red) outputs from a 100 µm diameter, 0.1 numerical aperture optical fiber coupled to a ZIVA Light Engine
The laser outputs are merged into a common optical train passing through an onboard metrology module and a despeckler to the light output port on the front panel (figure 1). The ZIVA and CELESTA Light Engines are distinguished by optimization for coupling into various optical fibers (table 1). This dictates the suitability of the ZIVA and CELESTA light engine for different applications.
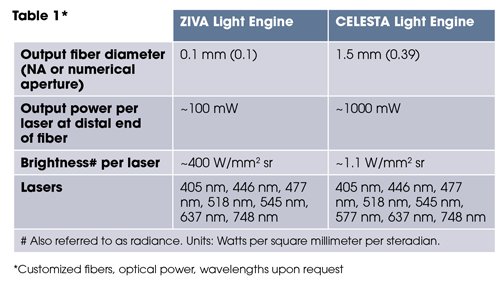
*Customized fibers, optical power, wavelengths upon request
The output of the ZIVA light engine is well-suited to structured illumination microscopy (SIM), stochastic optical reconstruction microscopy (STORM; figure 3) and other super-resolution microscopy techniques. In these applications, ZIVA provides an alternative to more costly and hard-to-align laser sources. The larger illumination fields produced by the CELESTA light engine are preferred for applications such as spinning disk confocal microscopy (figure 4) or MERFISH. MERFISH is an imaging technique that profiles cell populations based on identification of thousands of RNA transcripts per cell[1]. Identification is based on barcodes that are cumulatively assembled during multiple cycles of imaging and sample processing.
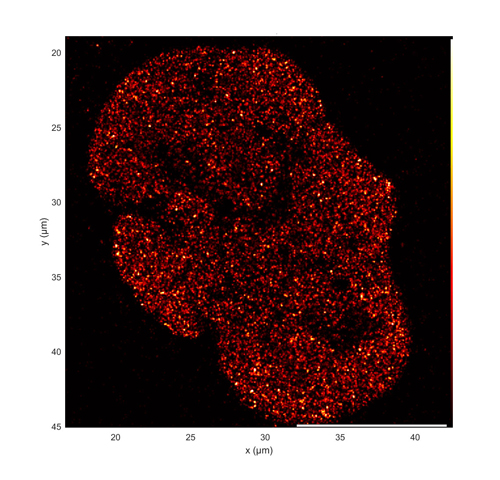
Figure 3. Super-resolution dSTORM image of the distribution of acetylated histone H3 (H3K27ac) in a single U2OS cell nucleus. H3K27ac is a marker for sites of active transcription. Image acquired using simultaneous 637 nm and 405 nm illumination from a ZIVA light engine
Acquisition of a complete data set may take several days [1], placing a premium on light source output stability. MERFISH requires a high intensity, spatially uniform illumination field matched to the dimensions (~200 mm2 ) of typical sCMOS camera sensors. To meet this requirement, Lumencor’s critical epi-illuminator coupled to the fiber output from the CELESTA light engine is installed in the microscope, providing about 500 mW/mm2 at the sample plane.
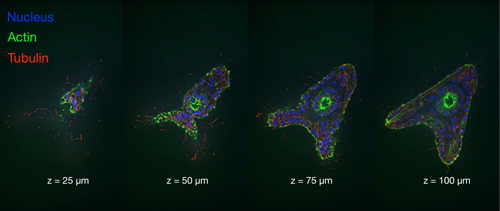
Figure 4. Four vertically spaced optical sections of a late blastula stage sea urchin embryo. Images acquired using a CELESTA Light Engine and a CrestOptics X-Light V2 spinning disk confocal scanner (Images courtesy of John Allen, Nikon Instruments, Inc)
References [1] JH Su, P Zheng, X Zhuang et al. Cell (2020) 182:1641–1659


