Optical technologies have revolutionised neuroscience over the past decade, offering a powerful new toolkit for researchers to explore the structure and function of the brain.
Ever since 2005, when Karl Deisseroth and colleagues at Stanford University first showed that light could be used to trigger the activity of neurons, scientists have been devising novel photonic probes that can be combined with genetic tagging to investigate the neural pathways that control our interactions with the outside world.
These optogenetic techniques achieve unprecedented precision by introducing light-sensitive proteins into specific cell populations, which generate a response when illuminated.
Various light-delivery mechanisms have been used to activate these genetically modified cells in the lab, but neuroscientists seeking to understand cognition and behaviour stand to gain more insight from experiments that can probe neural processes inside the living brain.
The most popular approach is to implant optical devices into the brains of freely moving animals, but the scattering and absorption of light in neural tissue requires an optical platform that provides enough power to excite the light-sensitive proteins, as well as the spatial resolution needed to stimulate specific regions of interest. Such neural implants must also be carefully designed for use in vivo, since they must be made of biocompatible, or at least inert materials, minimise tissue damage during insertion, and deliver enough light without overheating the surrounding tissue.
Optical fibres have in general become the technology of choice, since they offer the best compromise between optical performance and biocompatibility. The simplest option is to insert a single silica fibre into the brain of mice, an approach that has widely been used in optogenetics experiments over the last decade. But more invasive devices requiring larger fibres and higher power intensities are typically needed to reach the deeper parts of the brain, risking damage to the brain cells and neural processes that are being investigated.
One way to avoid that problem is to attach the implant to the surface of the cerebral cortex, rather than inserting it into the brain tissue. High power intensities are needed to penetrate large regions of the brain, and Monte Carlo simulations by Frederic Pain and colleagues at the Université Paris Saclay suggest that fibres with large diameters and numerical apertures can deliver the maximum amount of power without causing any thermal damage to the cortical tissue.
However, they also found that a single-fibre solution is unable to provide enough light to stimulate light-sensitive biomolecules located several millimetres away from the cerebral cortex. Instead, they propose a device composed of several silica fibres, each one coupled to a separate laser diode. ‘In this geometry, photons from each fibre can add up locally to ensure the fluence is above the activation threshold,’ the researchers note in their paper. ‘This divides the fluence required at the fibre tip by the number of fibres used in the device.’
Optogenetics allows neuroscientists to study neural processes by using an optical system to activate cell populations tagged with light-sensitive proteins. (Image: vitstudio/Shutterstock.com)
The team calculated that a device made from four silica fibres can modulate 300mm3 of brain tissue, while generating a temperature increase at the brain surface that is consistent with normal environmental factors or physiological activation. They also demonstrated the feasibility of the approach by constructing an optode made from off-the-shelf silica fibres and a surgical grid used in routine neurosurgery.
Such multifibre designs also open the door to delivering more complex sequences or patterns for optical stimulation, since each fibre can deliver light pulses of varying length, power and frequency. In contrast, conventional single fibres provide a uniform light distribution that cannot be changed without moving the implant, which is not ideal for in vivo experiments. Fibre bundles incorporating several thousand individual fibres have also been used to stimulate multiple areas of the brain with complex patterns that can be precisely defined using techniques such as computer-generated holography, but their large footprint, typically a few millimetres across, makes them difficult to insert and unsuitable for long-term implantation.
A less invasive approach that also enables depth-resolved optical stimulation is offered by tapered optical fibres, pioneered by researchers at the Centre for Biomolecular Technologies at the Italian Institute of Technology (IIT) in Lecce, working in collaboration with neuroscientists at Harvard Medical School in the US. These tapered fibres require only a single waveguide to be inserted into the brain, with the tip of the fibre gradually narrowed to a diameter of a few hundred nanometres to control the light emission and to reduce damage during insertion and operation.
Such tapered fibres can deliver light over large brain volumes, or to targeted subregions, with the depth of illumination ranging from a few hundred micrometres up to several millimetres. ‘A single tapered fibre inserted in a mouse brain can illuminate the entire depth of functional structures, such as the cerebral cortex or the striatum,’ says Ferruccio Pisanello, who together with colleague Massimo De Vittorio has led the optical engineering work at the IIT. ‘This allows a large number of neurons to be excited with a low power density, which reduces the amount of optical energy that is converted into heat.’
Dynamic and selective stimulation of specific cell populations is achieved by exploiting the modal properties of the narrowing waveguide, which makes it possible to control which region of the brain is being illuminated simply by changing the angle of the light when it enters the fibre. This allows multiple experiments to be performed without needing to move the implant, such as in a 2017 study that exploited a tapered fibre to excite different regions of the striatum in freely moving mice and observe the effects on their motor behaviour.
Such controlled light emission is achieved by coating the tapered end of the fibre with a thin layer of reflective metal, and then fabricating small windows along the depth of the implant to deliver a directed beam of light at specific locations. Windows of different sizes and shapes can be fabricated to deliver complex light patterns from the tapered edge and to control the extent of the illuminated region.
More recently, in 2019, the team extended the optical design to enable the detection of light generated by fluorescent indicators, offering a way to monitor neural activity during optical stimulation using a technique called fibre photometry. ‘Different wavelengths are needed for light delivery and collection, plus, for optical monitoring, the system must be optimised to capture as many photons as possible,’ comments Pisanello. ‘A single tapered fibre can detect neural activity in different parts of the mouse brain while the animal is performing a simple task.’
As far back as 2014, the IIT researchers created a spin-off company called OptogeniX to make its tapered-fibre technology more widely available for optogenetics experiments. While still small-scale, the company’s products are now starting to appear in research studies within the neuroscience community. ‘OptogeniX fibres are easier to implant and cause less tissue damage,’ comments Alfredo Fontanini, a neurobiologist at Stony Brook University in the US. ‘They also distribute light more evenly, so we can be confident we are activating or silencing our brain region of interest while diminishing the chance of photodamage.’
Pisanello and colleagues are now investigating whether it is possible to harness the photonic properties of tapered fibres to improve their performance as neural implants. Controlling light-matter interactions at the nanoscale might make it possible to implement more advanced techniques for label-free biomolecular sensing, such as surface-enhanced Raman scattering (SERS) – which is the main goal of a European project called NanoBright, being coordinated by Pisanello – and to find new ways to tailor both light delivery and collection in highly scattering tissue.
As an example, by patterning the surface of the tapered fibre with highly curved plasmonic structures less than 50nm apart, the researchers were able to create beamlets of light just 25µm across – comparable to the size of brain cells – that can be tuned over an angular range of 120° around the fibre. The nanopatterned fibres also allow specific Raman bands to be collected at arbitrary positions along the fibre, although this preliminary study was not yet able to find evidence of a SERS signal. ‘It is still very exploratory, but we are going in that direction,’ comments Pisanello.
Meanwhile, Antonello Cutolo of the University of Naples, working with Italian colleagues at the University of Sannio, has demonstrated a tapered multicore fibre that allows the output beam to be steered through 180° by altering phase and wavelength of light coupled into each of the cores. Each individual core acts as an optical antenna, leading to interference effects that can be exploited to control the direction of light emission and to illuminate multiple sites without needing to move the fibre. Multicore fibres with a diameter of 20µm can produce a beam width as narrow as 5µm, while larger regions can be illuminated by increasing the diameter and number of cores in the device.
Beyond these all-optical solutions, the IIT team has also worked with its collaborators to demonstrate a ‘fibertrode’ that incorporates a microelectrode just 10µm away from the light-emitting site. ‘Integrating the optical and electronic elements on the edge of the fibre makes it possible to stimulate neuronal activity and measure it at the same time,’ says Pisanello. ‘Such real-time feedback could offer a new paradigm in neuroscience, offering a way to study ongoing brain events such as plasticity or the consolidation of memory.’
By positioning the microelectrode in the dark spot just above an emission window, the researchers were able to avoid the photoelectric artefacts that would otherwise mask the electrical measurement. In its existing configuration, fibertrode was able to stimulate small volumes of brain tissue, equivalent to a few neurons, while the team believes that the same design concept could be extended to enable large-volume light illumination. Another key goal will be to optimise the design to enable simultaneous light collection. ‘Fibertrodes could be designed to record both the electrical activity of neurons and fluorescence signals from genetically-encoded functional indicators,’ points out Pisanello. ‘Dual-mode recordings of this sort would enable optical activation, electrical readout and fibre photometry within a single device.’
Despite the many advantages and opportunities offered by tapered optical fibres, the IIT team is well aware that other approaches might offer complementary capabilities for different optogenetic experiments. In the Deeper project, for example, an EU-funded consortium coordinated by De Vittorio involving 10 research institutions and two start-up companies, the aim is to create implantable optical devices for investigating the causes of brain disorders such as Alzheimer’s, depression and schizophrenia. ‘From the technology side we also need to enable neuroscientists to reach deep regions of the brain with the highest possible resolution,’ comments Pisanello. ‘Some experiments need wide-volume excitation, but others demand sub-cellular resolution to study specific neural circuits.’
One of the technology platforms being investigated in the Deeper project has the potential to offer higher resolutions, as well as more complex stimulation patterns. Rather than an optical fibre, this approach exploits an array of microscale LEDs (µLEDs) that can be addressed individually to enable optical stimulation from many different positions along a single probe. As an example, Keith Mathieson and colleagues at the University of Strathclyde in the UK have demonstrated a silicon-based array with 96 µLEDs that, when inserted into a mouse brain, enabled multi-point optical stimulation across the full extent of the mouse cortex at resolutions approaching the cellular scale.
More recently, they have created an optrode that combines a larger µLED device with an array of glass microneedles to deliver light to more than 180 sites in the brain – which could enable in vivo optogenetics experiments in large mammals.
‘We see µLEDs and tapered fibres as complementary technologies that could be used for different applications,’ says Pisanello. ‘The feeling is that µLEDs might offer higher resolutions, while tapered fibres are also able to offer fibre photometry. Our aim as optical engineers is to provide neuroscientists with the technology they need for each specific experiment.’
Sponsored: Solid-state illumination for all-optical electrophysiology
All-optical electrophysiology initiates and tracks electrical activity in excitable cells, such as neurons and cardiomyocytes using optically-actuated depolarization coupled with voltage-sensitive fluorescence detection.
It is implemented using optogenetic technology in tandem with imaging of voltage-sensitive dyes (VSD) or genetically encoded voltage indicators (GEVI). All-optical electrophysiology is suited to interrogating the spatial complexity of neuronal networks and identifying functional phenotypes associated with disease states.
Optogenetics and GEVIs are relatively recent developments. In contrast, VSDs have a 50-year-plus history of gradual improvement. VSD imaging is inherently challenging, requiring detection of small changes in small fluorescence signals. A functional VSD must be confined to the cell membrane, which constitutes about 0.1 per cent of the total cell volume.
Voltage-sensitive fluorescence signals result from the Stark effect – modulation of the dyes’ electronic configuration by the transmembrane electric field. The extent of modulation is weak, such that fluorescence changes are typically around 10% per 100 mV. Accurate measurements require very stable excitation light. LED sources and other solid-state lighting technologies excel in this respect. Fluorescent protein-based GEVIs generally produce smaller and slower voltage-dependent fluorescence changes than VSDs. Their primary advantage is the capacity for selective labelling of specific cell types. This capacity is important in intact tissues where adjacent cells may perform distinct functions. Furthermore, GEVIs and optogenetic actuators can be encoded in a single vector and co-expressed.
Solid performance
The illumination sources for all-optical electrophysiology applications must be capable of delivering two or more spectral outputs with millisecond temporal control. Solid-state light engines with either LED or laser sources can do this. Lasers provide higher irradiance in smaller areas and are preferred for in-vivo optogenetics applications due to their coupling efficiency into 200µm diameter multimode optical fibres.
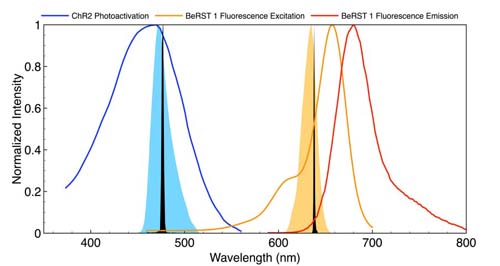
Figure 1
Spectral outputs for stimulation and VSD response detection must be spectrally well separated so that the relationship between stimulus and response is not distorted by optical crosstalk. A typical configuration is shown in Figure 1. Depolarisation is initiated by a brief pulse (5-50ms) of light, spectrally tuned to the absorbance or action spectrum of the optogenetic actuator channelrhodopsin (ChR2). The VSD (BeRST 1) is excited with red light, generating voltage-sensitive fluorescence responses of 24 per cent per 100mV.
Following ChR2 stimulation (475nm, 0.8mW/mm2, SPECTRA X Light Engine, Lumencor), simultaneous electrophysiological and optical recordings of membrane potential changes are effectively identical.
The capacity for independent control of light intensity and timing is particularly useful for controlling the extent of ChR2-induced depolarisation (Figure 2).
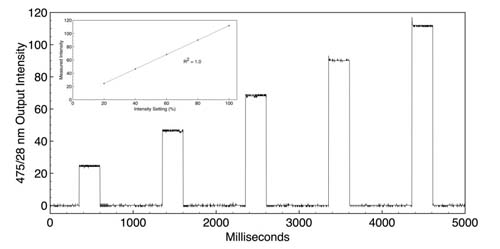
Figure 2
While electrophysiology remains a method of choice for small current and membrane potential detection in single neurons, the inherent challenges of electronic transduction relegate it to a somewhat limited scope of execution. Confounding electronic noise, challenging reproducibility and painstaking implementation are all commonplace in standard electrophysiology techniques driven by electronic transduction.
All-optical electrophysiology obviates such challenges with the use of optically actuated depolarisation and voltage sensitive fluorescence for detection. Light sources must be bright, stable, reproducible, spectrally discrete and ideally linearly dependent on drive current. Optical modulation must be robust on the millisecond timescale. Such lighting tools play an important role in the proliferation of such informative, bio-photonic cellular analyses.
References
DR Hochbaum, Y Zhao, AE Cohen et al. (2014) All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods 11:825–833
H Zhang, AE Cohen (2017) Optogenetic approaches to drug discovery in neuroscience and beyond. Trends Biotechnol 35:625–639
D Wagenaar (2012) An optically stabilized fast-switching light emitting diode as a light source for functional neuroimaging. PLoS One 7:e29822
YL Huang, AS Walker, EW Miller (2015) A photostable silicon rhodamine platform for optical voltage sensing.
J Am Chem Soc 137:10767–10776
JY Lin, PM Knutsen, RY Tsien et al. (2013) ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci 16:1499–1508
Further information