We all want a therapy to be available as soon as possible for Covid-19, and better testing approaches seem vital to end the lockdowns in place across the world. However, researchers must look deeper to find an effective long-term solution, said Michael Senior, business development lead at microscope producer Oni, based in Oxford, UK. ‘Understanding the basic biology and molecular mechanism behind this virus, and how it impacts cells, is going to be fundamental. And that’s where people are really interested in high-resolution imaging.’
In providing such imaging, super-resolution microscopy’s application in virology has been a success story for what are today increasingly well-established techniques and widespread instruments. Can it now help the world understand and overcome the Sars-Cov-2 coronavirus, the cause of the Covid-19 pandemic? Electro Optics sought answers from researchers using the techniques.
Jakub Chojnacki from the IrsiCaixa Aids Research Institute in Barcelona, Spain, noted that viruses such as HIV, influenza and Sars-Cov-2 are approximately 100nm in diameter. This is below the standard fluorescence microscopy resolution limit of around 240nm. Therefore, studying virus structures was once the domain of electron microscopy (EM), observed Chojnacki, which is ‘often difficult and expensive’. However, in recent decades scientists have exploited careful control of ‘on’ and ‘off’ states of fluorophores and knowledge of their precise location to penetrate fluorescence microscopy’s resolution limit. However, the different super-resolution fluorescence microscopy implementations mean that no microscope does everything well, Chojnacki noted.
One such technique is stimulated emission depletion (Sted) microscopy, which is typically the fastest technique for imaging structures below 100nm, such as viruses. Sted uses one focused laser beam that excites fluorophores and a second doughnut-shaped depletion beam that quenches fluorescence at the excitation spot’s edges. Scanning beams across samples builds up a high-resolution picture pixel by nanometre-scale pixel, although the intense light can photobleach the fluorophore.
Perhaps the other two most prominent super-resolution methods are stochastic optical reconstruction microscopy (Storm) and photoactivated localisation microscopy (Palm). Both use lasers to randomly activate and locate small numbers of fluorophores to progressively compile super-resolution images. ‘Because of the reconstruction times, you cannot really look at very fast events,’ Chojnacki said. Consequently, Storm/Palm techniques usually apply to fixed, inactive samples when studying viruses.
To be investigated, virus components must be tagged with fluorescent antibodies, for example, which Chojnacki did not find a major difficulty when using Sted. ‘Importantly, when using Sted, you maintain the potential for live cell experiments,’ he said. This ability is important ‘because virus entry and virus assembly are not a series of discrete steps, they are a series of overlapping dynamic events,’ he added.
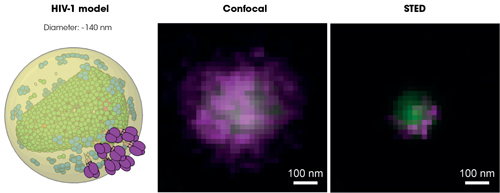
Individual HIV-1 particle imaged under confocal and super-resolution fluorescence microscope. Green and magenta colours represent virus internal and surface proteins, respectively. Credot: Jakub Chojnacki, IrsiCaixa
Sted becomes even more powerful when combined with fluorescence correlation spectroscopy (FCS), which analyses variations in fluorescence and interprets the mobility of molecules studied. While working in Christian Eggeling’s lab at the University of Oxford, UK, Chojnacki exploited it with colleagues at the Institute of Research in Infectious Diseases (IRIM) in Montpellier, France. Together they used this approach to study the lipid envelope surrounding some viruses in detail. ‘We have established, in live cell context, how HIV prepares a favourable lipid environment for virus assembly in the plasma membrane,’ he said. ‘This was not really appreciated before when studying viruses in fixed samples.’
Building on HIV findings
Chojnacki’s IRIM collaborator Delphine Muriaux added that they discriminated virus assembly platforms that were 50 to 80nm in size on immune cell surfaces. ‘We could show that the virus is able to segregate certain lipids and not others during its formation at the cell surface, in a live single cell,’ she said. ‘This is incredible. Sted/FCS allowed us to work in a single virus platform at the cell surface of a single cell. This is the nanovision of viral life.’
Using Palm microscopy, the IRIM team was also able to visualise HIV assembly in immune cells molecule after molecule. Muriaux’s colleague Cyril Favard said the team then exploited a Bayesian framework method developed by Jean-Baptiste Masson at the Pasteur Institute in Paris, France. This provides robust statistical classification of either short trajectories of single molecules, or longer trajectories with missing steps. ‘In our case, it is used to identify viral proteins that will self-assemble and to follow the real-time generation of a new 100nm-diameter HIV-1 virus at the single molecule level,’ he said. In the future, he expects correlative microscopy, combining optical methods with other techniques like electron microscopy or atomic force microscopy to provide more insights.
The IRIM team is now planning to study Sars-Cov-2 using the same capabilities. Both Sars-Cov-2 and HIV are very close in terms of particle sizes, Muriaux stressed. They are around 35 to 140nm in diameter, and as such cannot be seen by classic optical transmission. Sted and Palm can be used for structural purposes with antibody labelling on fixed samples, both with around 20nm precision, but with different chromophores, she stressed. While Palm can follow single proteins in living samples, there is no equivalent for lipids, added Favard, which is why the team switched to Sted-FCS. ‘We can transpose some of the labelling strategies that we have used for HIV-1 to Sars-Cov-2, and have developed others,’ Muriaux said. ‘Both viruses are RNA-enveloped viruses with some differences, but might share common features.’ The team is studying Sars-Cov-2’s mobility in solution, where it goes inside cells, and how many viruses there are within cells.
Joe Grove’s team at University College London (UCL) is similarly using microscopy to measure ‘as many things as possible as we can about viruses’, Grove said. ‘The proteins on the surface of viruses like HIV – like the coronavirus – are molecular machines. Understanding how they move gives us fundamental insight into how they work.’
He highlighted the importance of what can be learned by using optical microscopy to look at single particles. ‘Some viruses are very regular and behave in somewhat predictable ways,’ Grove noted. ‘But other viruses are more diverse in their morphology and constituents. We could use biochemical methods to investigate some of these things, but you completely lose the single particle information. You can also access single particle information using electron microscopy. But if you wanted to try and quantify numbers of proteins or identify specific proteins, EM is not particularly well suited to that.’
When viruses and antibodies meet
The specific areas that Grove’s team studies include how antibodies interact with virus particles. ‘We’re also interested in the dynamics and behaviour of the proteins on the surface of virus particles,’ he explained. ‘Optical microscopy really is pretty much the only tool that we can use to start probing these features.’
The UCL team can immobilise virus particles for single molecule and super-resolution imaging in total internal reflection fluorescence (TIRF) microscopes, which enable them to study specimens less than a micron thick. ‘We can look at antibody binding in real time using very sensitive quantitation methods,’ Grove explained. ‘The viruses are fluorescently labelled, as is the antibody. But 99 per cent of the antibody has fluorescent label A and one per cent label B. By looking at the frequency of virus particles that are positive for the minority label, we can use statistical modelling to infer the number of antibodies that are on the virus particles.’ Such techniques can illuminate key information about viruses, such as how many antibodies need to bind to a virus before that virus is no longer infectious.

Joe Grove’s (UCL) team uses analysis of fluorescently labelled viruses and antibodies to measure the intensity of antibody bound to virus. Credit: Joe Grove/UCL
This approach could provide information of interest about Sars-Cov-2. ‘How do viral entry proteins work?’ Grove asked. ‘How do antibodies stop them from working? We are interested in applying some of our single particle analysis approaches to Sars coronavirus. Any technologies that have been developed to allow high-resolution imaging of virus X can be applied to virus Y, and there will be people the world over considering how they can apply their optical techniques to Sars.’
Yet Grove is currently unable to work directly with Sars-Cov-2, in part because that requires biosafety containment level 3 (BSL-3), and his labs have BSL-2 facilities. Instead, his team intends to work with pseudo viruses. This might involve combining proteins from the surfaces of the Sars-Cov-2 virus with replication-defective HIV or another retrovirus. ‘We’d be interested in comparing Sars-Cov-2 to Sars-Cov-1. We’re hoping to use basic biology and computational simulations, but then ultimately, we would need the microscopy to confirm some of these computational simulations.’
Producing the Nanoimager instrument, capable of super-resolution microscopy and other cutting-edge techniques, Oni is busy trying to support researchers studying Sars-Cov-2. The Nanoimager looks unlike most other microscopes, taking the form of a compact black box requiring no extra specialised equipment. ‘There are a lot of things that people do not need on a normal microscope,’ said Ricardo Bastos, Oni’s head of applications. ‘Simplifying it made it stable. Making it stable and robust allowed us not to have optical tables and dark rooms.’
Into the biosafety lab
Oni’s researchers are working on collaborations with researchers at a BSL-3 lab at the University of Oxford. This includes trying to develop an improved diagnostic test. The Nanoimager’s design makes it well-suited to biosafety laboratories, explained Bastos. ‘Because of airflow, there’s constant vibration. I’m trying to look at a virus, vibration is a bad thing. Our system can counteract that vibration. Then in the BSL-4s, the big hardcore safety rooms, you’re in a big suit. Imagine trying to switch on 50 buttons! [The microscope] has to be something simple and with no sharp corners, which is not too complex. That’s why, typically, researchers use old microscopes. But we can now give the tools of the most advanced microscopy to these people, because it’s such an easy and robust system to use.’
Bastos highlighted other virology studies that show what might be possible with Sars-Cov-2. ‘We know that viruses infect cells, they take advantage of the host machinery to start replicating,’ he said. Bastos explained that Stephan Becker from the University of Marburg has found that Ebola viruses use the network of actin filaments on which other proteins move through cells to make and move new viruses. ‘You could do a similar approach with Sars-Cov-2, trying to understand the key components of the cell it interacts with, and trying to develop drugs that prevent this interaction specifically,’ Bastos said. ‘So you prevent the virus from replicating once it infects a cell.’
In Barcelona, Chojnacki and colleagues are planning to use super-resolution microscopy to study delivery of drugs for Sars-Cov-2 encapsulated in nanocages into cells. ‘We want to have a look how well these 100nm-sized nanocages can deliver these drugs to the cell interior,’ he said. He and his colleagues at the nearby Comparative Medicine and Bioimaging Centre (CMCiB) are also trying to set up a BSL-3 super-resolution microscopy unit. ‘Such a facility would enable us to perform advanced microscopy experiments even in the live infectious pathogens such as Sars-Cov-2,’ Chojnacki said. ‘In the future we will have the possibility of rapidly studying such emergent pathogens in a fully infectious context.’
Further advances in super-resolution microscopy can move towards live cell capabilities able to observe the behaviour of viruses in tissues, Chojnacki hopes. ‘Laser light penetration and optical aberrations are problems moving into the tissues,’ he explained. ‘But with the increasing use of adaptive optics, scientific questions in the context of tissues will actually be able to be addressed.’
But Chojnacki is clear that technology that’s already available can yield valuable insights: ‘Approaching Sars-Cov-2 with super-resolution microscopy might yield a new layer of insight into the structure of the virus, or how the virus buds or how the virus enters the cell,’ he said. And even looking at already well-studied viruses with the technology may pay dividends. ‘Those insights may offer new avenues for treatments or vaccines.’
Filtering in fluorescence
Detecting weak light signals is the cornerstone of fluorescence microscopy, in which filters play a key role
Although they make up just a small part of a complete instrument, the requirements for optical filters in fluorescence microscopes are huge. That’s because they must separate and focus light in a way that allows the detection of fluorescence signals a million times weaker than the light a sample is illuminated with. Emitted light is 1,000 times weaker than scattered light, which in turn is 1,000 times weaker than the illumination light.
In fluorescence microscopy, fluorescent dyes (fluorophores) absorb light of a certain wavelength and almost instantly radiate light of another wavelength – a process known as excitation and emission. The goal of the microscope is to separate emitted light (dim) from excitation light (bright).
The wavelengths at which most fluorophores absorb or emit light are closely located and often even overlap. This requires that the excitation and emission filters used within microscopes have very steep edges to effectively separate absorption and emission peaks from one another. High out-of-band rejection is essential to make the weak fluorescence signal detectable among the strong illumination light.
The dichroic beamsplitter is the third type of filter required in fluorescence microscopes, which has a unique coating that reflects excitation light, but transmits the emitted fluorescence. This allows the illumination light to be reflected into the microscope lens and onto the sample, while at the same time the fluorescence light to pass through into the eyepiece or onto the CCD or CMOS camera attached to the microscope.
Well-designed dichroic beamsplitters have broad reflection and transmission wavelength ranges with a sharp transition in between. They help suppress the excitation light by at least two orders of magnitude and allow contrast rich imaging of cells and other specimens.

Classical fluorescence filters (circular) for wide field microscopy and rectangular CVFs for confocal microscopy
Around 1970, Delta Optical Thin Film was the first to suggest the use of interference filters to Carl Zeiss and Leica, and since then its high-performance fluorescence filter-sets and later on continuously variable filters have been used in numerous groundbreaking fluorescence microscopes. This includes Leica Microsystems’ SP8 Dive Deep In Vivo Explorer.
The SP8 Dive Deep In Vivo Explorer is the first spectrally tuneable solution for multicolour, multi-photon imaging. The instrument is equipped with 4Tune, which is a tuneable, non-descanned detection system. This provides the users with flexibility and the opportunity to develop innovative multicolour deep in vivo experiments.
The 4Tune detector can be equipped with two to four detection units and it can be configured with hybrid detectors and photomultipliers. It uses a combination of continuously variable dichroic beam splitters and continuously variable edge filters to separate the emission light. These optical filters were developed in close collaboration with Leica Microsystems’ development team by Delta Optical Thin Film, and comprise improved and tailor-made Continuously Variable Short Wave Pass and Continuously Variable Long Wave Pass Filters, and a Continuously Variable Dichroic Beamsplitter that are optimised for their application in confocal microscopy.
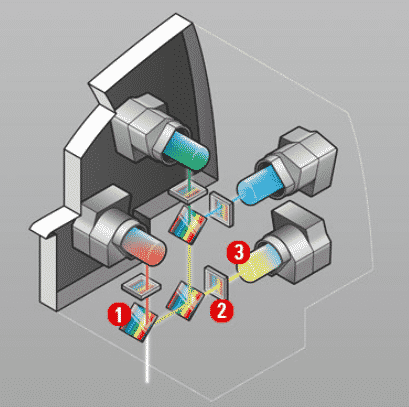
4Tune detection system: 1) Variable dichroic beamsplitter 2) Variable bandpass filter 3) hybrid detectors or photomultipliers. Credit: Leica Microsystems
The combination of continuously variable long wave and short wave filters enables the construction of bandpass filters, which can be tuned continuously between 380nm and 800nm. This feature within Leica’s microscope means microbiologists can freely tune their detection range over the whole visible spectrum.
The spectral properties of Delta’s Continuously Variable Filters (CVFs) vary continuously along one dimension of the filter. The coating thickness increases continuously and the cut-on wavelength changes continuously along it. Thanks to this feature, a single CVF can replace a number of fixed filters in an instrument, and the centre or edge wavelength can be adjusted by sliding the optical filter.
Fine coatings
Thin film coatings are essentially what give a lot of the properties of the filter, notably the high transmission in the desired wavelengths, out-of-band blocking and the steep edges that separate absorption and emission peaks from one another. The advances in coating deposition methods over the last 20 or more years have been one of the big enablers to making high performance filters.
All continuously variable filters from Delta Optical Thin Film are coated with ultra-hard surface coatings, which are produced with an advanced plasma process that builds layers with a much higher packing density than traditional hard coatings. The result is optical filters that set new standards in edge steepness and transmission level, and also provide improved blocking. Coated on a single fused silica substrate, these optical filters exhibit minimal auto-fluorescence and a high threshold to laser damage. A prerequisite to this success was the development of software to design CVFs and the necessary tooling, as such software tools are not commercially available.
Delta began volume manufacturing fluorescence filters in the early 1970s and were among the first to implement computer-controlled deposition in the early 1990s. Using its own synthesis and deposition control software and proprietary optical monitoring equipment allows the company to offer coatings made with plasma assisted e-beam evaporation technology with spectral properties comparable to that achievable by ion beam sputtering (IBS) and magnetron sputtering at attractive prices in high quantities. With its decades of experience, today, Delta is one of the largest suppliers of high-performance fluorescence filter sets to the market-leading manufacturers of fluorescence microscopes.