Given that today one of the most important ways of diagnosing cancer relies on coloured dyes, optical physics has a great opportunity to improve it.
Typically, pathologists study cancerous tissues under microscopes using two stains, haematoxylin and eosin. This histopathological method, better known as H&E, turns different parts of cells blue and pink.
‘In a pathological lab, the traditional H&E method is used to determine whether the cell or tissue is cancerous,’ explains Xudong Fan, professor of biomedical engineering at the University of Michigan. ‘However, H&E does not provide much information about the molecular activities of a cell.’ Beyond H&E, DNA and antibody-based dyes can identify cells’ molecular activities via fluorescence imaging, Fan added, but this still comes against background fluorescence ‘noise’. ‘The difference in the fluorescence intensity between the normal cancerous cells and tissues is small,’ Fan said.
Fan’s team is therefore among many scientific teams seeking to improve cancer diagnosis. Some, like the Michigan group, seek to use optics to improve H&E methods in tissue removed from patients’ bodies. Others seek to provide entirely different approaches, using optics to power non-invasive liquid biopsies.
For example, rather than using traditional fluorescence emission in tissue biopsies, Fan’s group uses laser emission. The Michigan team stains cell nuclei with dyes that, inside cancer cells, emit slightly more light than normal cells. ‘The idea is to place the cells and tissues between two mirrors that form an optical cavity and provide optical feedback, which can significantly enhance the small difference in the optical signal between the cancer and normal cells,’ he explained. ‘When the external pump is above the lasing threshold, strong laser emission from cells and tissues can be observed. The optical feedback provided by the mirrors enhances such a small difference. For example, we found the lasing emission difference can be as large as 10 to 100 times, whereas fluorescence difference is about 50 per cent in most cases.’
The strength of laser emission is not the only difference. ‘We found that cancer cells have lower lasing thresholds than the normal cells,’ Fan said. ‘If we set the external pump at the level between the two lasing thresholds for cancer cells and for normal cells, we can make most of the cancer cells lase, whereas normal cells do not lase.’ Accordingly, the Michigan team clearly distinguishes cancer cells from normal cells, and cancer tissue from normal tissue in lung, colon, breast, and stomach tissues, in frozen and formalin-fixed paraffin-embedded forms. ‘Additionally, since the laser emission capability is related to cell molecular activities, rather than morphology, we can pick up earlier signals indicating early progress of cancer cells that are difficult to pick up by H&E,’ Fan added.
Related stories: Stargazing optics to spot cancer early
How multi-line lasers simplify and help commercialise biomedical imaging systems
Predictably, this approach brings new challenges with it. ‘Optical alignment becomes more important, as the lasing signal depends on how well you are able to align the two mirrors,’ Fan said. ‘In the future, an automation approach may help eliminate this issue.’ The Michigan team has already built a fully automated system, which scans 2 x 2cm of 5 to 10µm thick tissue in about 10 minutes. They are now working with hospitals to image more tissue samples.
Such laser emission microscope (LEM) processes are highly compatible with the routines in a pathological lab, Fan stressed. ‘A technician can simply treat the mirror as a glass slide they routinely use,’ he said. ‘Currently, we have two LEM systems built that will be placed in a pathological lab.’ The accuracy of the tests can be tailored, Fan added. ‘In practice, sensitivity and specificity can be adjusted by clinicians. Some prefer to have low false negatives, but more tolerate higher false positives.’
Guided by spectroscopy
Viacheslav Artyushenko, founder and chief technology officer of Art Photonics in Berlin, is seeking to detect tumour margins using label-free fibre spectroscopy instead of H&E stains. Surgeons struggle to identify the border where tumours end and healthy tissue begins. His company therefore develops fibre probes to enable spectroscopic distinctions of the tumour margins during operation. ‘It's fast and can give answers to the surgeon in real time,’ Artyushenko said.
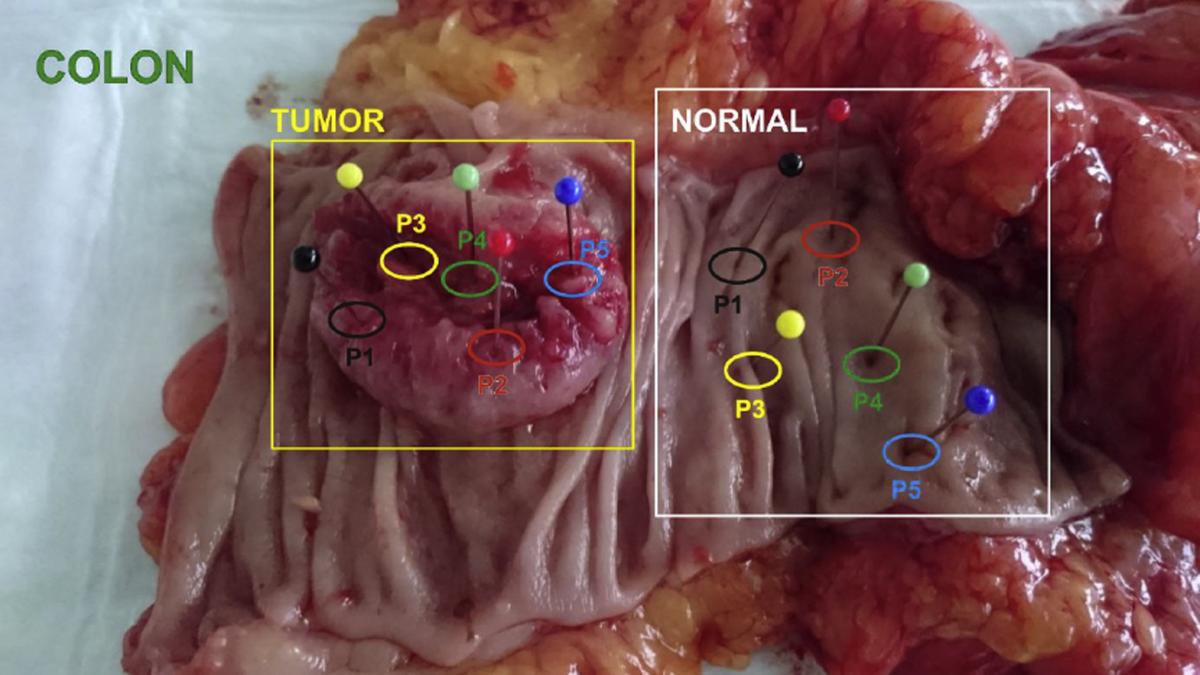
Art photonics and its collaborators use distinct points in malignant and benign colorectal tissue to develop a combined fluorescence/absorption spectroscopy technique for cancer diagnosis
Art Photonics’ expertise is in speciality fibre optic production. It uses different fibre types to produce spectroscopy probes exploiting a very broad spectrum range from 300nm ultraviolet light up to 16µm infrared light. The four main spectroscopy methods currently on the market are: mid infrared (IR) absorption; Fourier transform IR (FTIR); near IR-diffused scattering and reflection; and Raman scattering and fluorescence. ‘For each method, you need a specific fibre-optic probe design,’ Artyushenko explained. In particular, he highlighted the mid-infrared range with wavelengths from 2 to 16µm, known as the ‘fingerprint region’. ‘It includes absorption bands of almost all key vibrations of organic molecules and creates a chance to analyse tissue composition precisely.’
Artyushenko’s company has therefore created a ‘universal multispectral fibre system’. Developed together with Technical University Berlin researchers, and part-funded by Investment Bank Berlin, the Fibre Optic Molecular Sensor, or FOMoS, combines all four methods in one mobile prototype. ‘We can go to an operation room and compare all spectral methods,’ he explained. This allows researchers to compare each method’s performance on key medical parameters: sensitivity, specificity, and accuracy. ‘Each method has pros and cons,’ Artyushenko said. ‘But we have a chance now to select the best one – or to combine two or three through data fusion.’ He noted that FOMoS is not a commercial product, but a way ‘to develop specific tumour sensors for different organs’.
Spectroscopy characterises cancers based on different biomarkers. For example, glucose is ‘the fuel to build more cells in tumours,’ Artyushenko said. ‘Normally at the aggressive front of growing tumours we see high concentrations of glucose,’ he explained. ‘We see it in the infrared range of 8 to 11µm.’ However, although it is developing instruments for use in hospitals, Art Photonics is ‘not going in to educate surgeons about spectroscopy principles’, Artyushenko added. ‘Chemometric software for mathematical treatment of spectra should provide some kind of real-time signal,’ he said. Options include coloured lights, or even sounds ‘like the beeps when you park your car’.
Molecular biomarkers also offer opportunities for detecting early-stage cancers, explained Coen van Kalken, chief executive officer at Qurin Diagnostics, Amsterdam. The company’s focus is using urine as a ‘liquid biopsy’. ‘Cancer biomarkers in urine are present at extremely low concentrations, so there is a need for highly sensitive measurements,’ van Kalken said. ‘Photonic biosensors can offer ultra-high sensitivity, ease-of-use, versatility of molecular targets, robustness in various sample matrices and, ultimately, low-cost diagnostic tests.’
Qurin has identified appropriate DNA, or protein biomarkers, for non-invasive and early diagnosis. ‘Qurin’s primary goal is detection of specific epigenetic modifications,’ van Kalken said. For this approach, identification of strong biomarker panels with high diagnostic value is critical, he added. Qurin has identified the panels using advanced data mining algorithms from the financial industry, in collaboration with big data company Grace Systems, in nearby Utrecht. AI approaches significantly shorten the time needed to make these panels available for clinical evaluation.
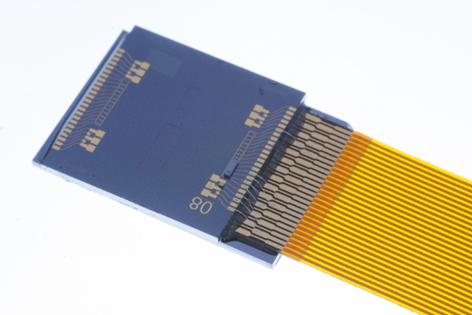
A photonic biochip produced by LioniX for Qurin’s liquid biopsy biosensor
The company has exploited its biomarker selection to develop a versatile biosensor using innovative nano-coatings developed by Surfix, in Wageningen. Last April, Qurin jointly acquired Surfix with LioniX International, also in The Netherlands, in Enschede. LioniX contributes photonic devices built on a mature silicon nitride platform that enables highly sensitive biosensors that measure biomarkers using evanescent field-based sensing with Mach-Zehnder interferometers.
‘Bio-recognition elements, such as complementary DNA, are immobilised on the surface of the waveguides, allowing hybridisation reactions to take place in the evanescent field,’ said van Kalken. ‘Upon hybridisation of target DNA, the refractive index at the waveguide-solution interface changes, causing the measured interference pattern to shift. This signal is translated to a concentration measurement by appropriate calibration.’ The current photonic chips with multiple Mach-Zehnder interferometer structures in silicon nitride enable detection of up to six analytes simultaneously, sufficient for a typical biomarker panel for one type of cancer.
Both LioniX and Surfix have refined their technology over the past decade to enable a solid basis for biosensor development, explained van Kalken. The European BioCDx consortium has worked on further integrating these sensors in microfluidic cartridges. This will enable a prototype readout instrument and demonstration of ultra-sensitive protein measurements at clinically relevant concentration levels. The Netherlands-based researchers will present the results at the upcoming Photonics West, in the Integrated Optics: Devices, Materials, and Technologies XXIV conference on 4 February during Session 7.
‘Qurin aims to build on these efforts and to develop a diagnostic instrument with low-cost test cartridges in which our disease-specific biomarker panels are integrated,’ van Kalken said. ‘A prototype biosensor for the first selected medical application will be developed in the coming period. Major efforts are directed to further increase signal-to-noise, reduce effects of drift and reduce cost price per test through further sensor miniaturisation and multiplexing. In addition, optical interfacing and the combination with fluidics require attention to implement an easy-to-use, robust and cost-effective integrated system that can be used at the physician’s office.’
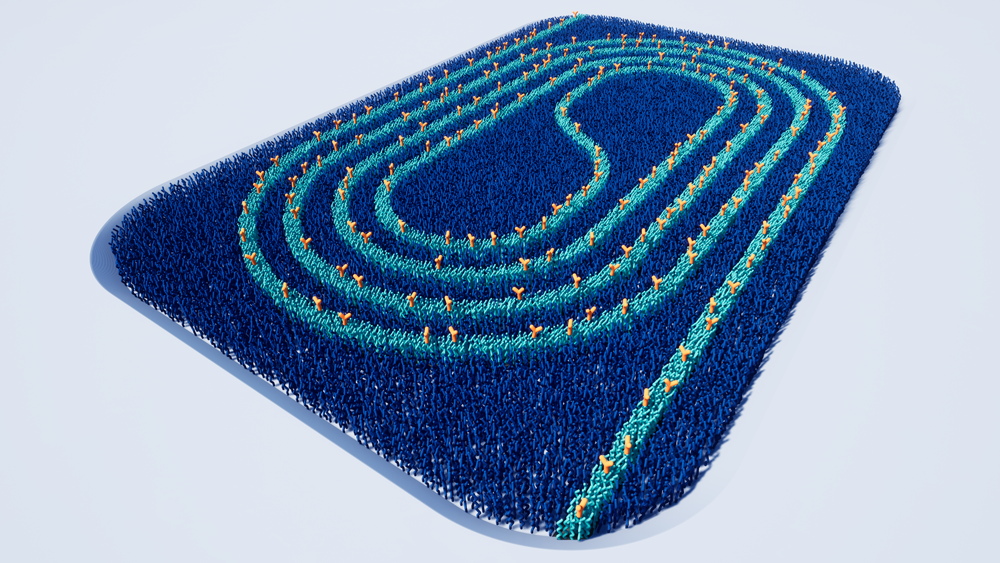
Surface chemistry applied to photonic waveguides to enable biosensing in liquid cancer biopsies
Finding where to stop
Another class of biomarkers is the ratio of fatty lipid molecules to water, which differs between normal and cancerous cells, noted Artyushenko. Water concentrations are higher in breast tumours or oral cancers, for example. ‘You can detect the difference by near infrared spectroscopy in the range from 1 to 2µm. You can also see it better by Raman spectroscopy in the so-called high wavenumber region.’ This is a highly specific and accurate biomarker for oral squamous cell carcinomas.
Exploiting this, Art Photonics was invited by its two partners – skin sensor firm RiverD and the Erasmus Medical Centre, both in Rotterdam – to set up a company called SurGuide. ‘Cancer surgeons basically work blind,’ explained Gerwin Puppels, RiverD’s chief executive officer. Locating oral cancers currently involves radiology approaches like ultrasound, coherence tomography (CT), and magnetic resonance imaging (MRI). ‘The radiologist walks with the surgeon to the operating room and at the door gives him the pictures in his hand, wishes him good luck and then leaves,’ Puppels said. ‘It might be a month later, the tumour might have grown or be in a different position. Basically, the surgeon only has his or her hands and eyes to determine where he or she will cut and where to stop.’
In oral cancer cases, tumours are in places like the patient’s tongue or the floor of their mouth. The target is removing the whole tumour and a further 5mm margin of the tissue. Only 15 to 20 per cent of oral cancer cases achieve that, Puppels and colleagues have found. ‘It's obvious that the hands and eyes of the surgeon are not sufficient,’ he said. The MarginGuide product that SurGuide is developing inspects the tissue that has been removed by the surgeon. It drives a fibre optic needle, through which Raman spectra can be taken, into the tissue. ‘Within 10 to 15 minutes, you can inspect the tissue in many locations,’ said Puppels. 'Frequently, the surgeon has actually cut through the tumour. We simply mark this and tell the surgeon exactly where to take extra tissue to make sure that an insufficient margin becomes a sufficient margin.’
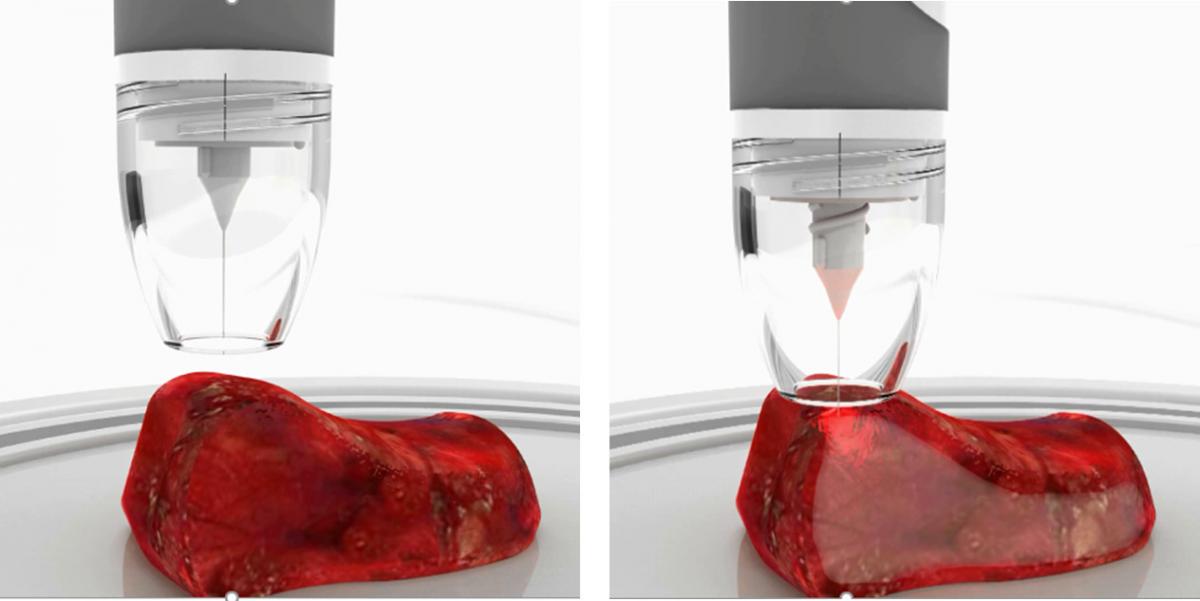
Art Photonics provides fibre-needle probes for ex-vivo tissue analysis realised by MarginGuide for tumour margins detection during operation
Like the technology from Qurin and the Michigan team, this approach is still early in the commercialisation process. But MarginGuide is perhaps the furthest advanced. And although oral cancer is not the biggest indication, with around 60,000-70,000 operations per year in Europe, the problem is pressing, according to Puppels: ‘It is probably the surgery with the worst score,’ he said.
‘We have indications that, where you now barely have 20 per cent of adequate surgeries, we might be able to improve this to over 50 per cent, thanks to the intraoperative feedback to the surgeon.’
He hopes to develop MarginGuide into a commercially available product over the next two years. A key step will be attaining the CE mark indicating conformity with relevant EU directives, which may happen in early 2022. ‘We will certainly extend this to other cancer surgery indications in the future,’ Puppels added. ‘We really want to establish this as a technology platform.’


