Raman spectroscopy is a non-destructive chemical analysis technique that is well-established in various fields such as pharmaceuticals, life sciences, carbon materials and geology and mineralogy.
Because it allows for label-free characterisation of biochemistry without damaging samples, it has enhanced many areas of biology research. However, Raman spectroscopy has not yet made its way into hospital laboratories, which, if made possible, could potentially transform clinical diagnostics.
Researchers from the Leibniz Institute of Photonic Technology (IPHT) in Jena, Germany, are developing tools that could allow human tissue to be analysed on a cellular level, without the need for a biopsy. This could provide surgeons with a means to distinguish between cancerous and non-cancerous tissue without having to wait for separate laboratory analysis of tissue sample. The instruments would complement existing pathological techniques, allowing for more reliable and faster disease diagnosis.
Getting Raman-based techniques into the clinic has many difficulties, including ease of use, sensitivity, standardisation and making sure the techniques can be integrated with existing methods.
‘Many of the techniques being employed in clinical applications today have been well-established in chemistry and physics circles for decades,’ Dr Sarah Locknar, staff scientist and technical business development manager at Omega Filters, explained. ‘The biggest barriers are familiarity with the techniques, which are not necessarily known by most biologists, and establishment of validated protocols that use the techniques and have been proven effective by comparison with known standards.’
Although the potential of Raman-based methods in clinical diagnostics has been proven in many studies, there needs to be more thorough research, added professor Fiona Lyng, of the Centre for Radiation and Environmental Science (RESC) at Dublin Institute of Technology. ‘Most of the studies published to date have used small sample sizes so these need to be increased to really show the potential for clinical diagnostics,’ she said. ‘Standardisation is important for sample preparation, spectral acquisition, data pre-processing and data analysis, so that multi-centre trials can be carried out.’
Lyng pointed to work by the European project, Raman4Clinics, addressing these standardisation issues for Raman spectroscopy. The programme is funded by Cost – European Cooperation in Science and Technology, a framework supporting trans-national cooperation among researchers, engineers and scholars across Europe.
Raman4Clinics fosters collaboration between biophotonics research clusters and physicians or clinicians. ‘It advances the conversion of scientific findings into clinically relevant, practicable and economically feasible diagnostic methods and systems, setting up a technology portfolio based on Raman spectroscopy,’ Lyng said.
One of Raman4Clinic’s research projects involved a clinical utility study of Raman spectroscopy for cervical cancer screening on Pap smear samples from 1,000 patients. This is funded by the Health Research Board in Ireland and carried out in partnership with the Coombe Women and Infants University Hospital.
‘We are also extending this work on cytopathology to other cancers, such as oral, lung and thyroid cancer,’ commented Lyng.
Raman in pathology
Researchers from IPHT, led by scientific director professor Jürgen Popp, are developing Raman approaches that could provide rapid, non-invasive pathological analysis of cells and tissues as surgeons operate.
‘We have developed a compact laser-scanning microscope aimed at the label-free and fast intraoperative online analysis of tissue sections for histopathologic assessment of disease-associated molecular changes,’ said Popp.
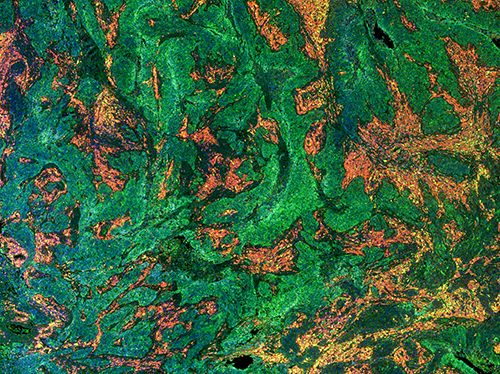
For example, the spectroscopic method will allow tissue samples to be analysed to determine tumour type and grade, and provide reliable delineation of tumour margins.
Currently, pathological staining procedures represent the gold standard for the detection of tissue pathologies in biomedicine. However, tissue staining is constrained to ex vivo investigations only, and the imaging speed and number of molecular labels are limited. In this context, Raman spectroscopy could enable minimally invasive and label-free imaging of a wide range of specific molecular structures.
IPHT’s work focuses on the development of sensitive spectroscopic technologies that will work with pathological staining protocols and can serve as a screening tool in future clinical routines, reducing the pathologist’s workload.
While the advantage of Raman spectroscopy is its high specificity, it suffers from its poor sensitivity, which can be overcome by using non-linear coherent Raman scattering (CRS), Popp explained: ‘In particular, in combination with further non-linear spectroscopic imaging modalities [such as] two photon excited fluorescence (TPEF) and second harmonic generation (SHG), CRS enables the label-free detection of changes in tissue composition and morphology based on virtually any molecular marker at high speed, ideally suited for intraoperative histopathology.’
Traditionally, Raman techniques require complex, maintenance-intense and expensive instrumentation, which hinders the routine application of multimodal non-linear imaging in biomedicine.
Hence, to allow for clinical translation, IPHT’s tool is a highly integrated, multi-contrast non-linear microscope, which involved the development of a novel high-power ultrashort fibre laser source that can be used for 24/7 maintenance-free operation.
The instrument’s operation was intentionally kept simple for use by pathologists and surgeons. It can complete the results from established staining procedures by complementing images of the distribution of specific molecular targets in tissue, providing a label-free all-optical pathology of tissue specimen.
‘This microscopic platform can be combined with laser tissue ablation for tissue-specific laser surgery,’ Popp explained. ‘The specific detection of malignant tissue during curative surgery is the most important precondition for complete tumour removal.
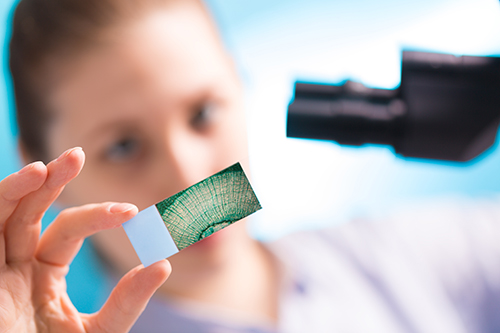
‘To further extend the applicability of this multimodal imaging approach for in vivo tissue screening of difficult-to-access body regions, an imaging fibre probe has been recently developed.
‘Currently, we have achieved a stage of development beyond mere basic feasibility. However, we are far from being integrated into routine clinical diagnostics. We currently pursue this important goal – the translation of our approaches into clinical diagnostics.
‘In other words, optical spectroscopic approaches have proven their potential to certain diagnostic issues in numerous proof-of-principle tests; however, their actual effectiveness needs now to be tested under routine clinical conditions in patients, or samples in the form of comparative studies.’
Popp added that the developed spectroscopic approaches need to be evaluated on a broader patient cohort in a multi-centre preclinical study to account for inter and intra patient variations with respect to the histopathological gold standard. In addition, the techniques need to be certified, which requires the support of industry or appropriate funding.
Finally, yet importantly, physicians, in particular pathologists, need to be convinced of the usefulness of the new approaches to complement their established gold standard devices. In parallel to clinical studies, negotiations with health insurance concerning reimbursement arrangements of costs are required.
Although it is clear that further research and development and industry support is needed before Raman-based approaches exist in clinics, the benefits are numerous.
With point-of-care diagnostics becoming ever more important to deal with global healthcare challenges, Raman methods provide a promising way of reducing the time and costs associated with current diagnostic techniques, whilst improving reliability.
Confocal fluorescence imaging for breast cancer diagnosis
The translation of biophotonics technologies into clinical environments requires collaboration between research and industry. Optical filter manufacturer Omega Optical is working with the University of Vermont on testing the feasibility of confocal fluorescence imaging for diagnosing breast cancer.
‘We are trying to determine if we can use intrinsic fluorescence to distinguish healthy from cancerous or precancerous tissues at the microscopic level,’ said Dr Sarah Locknar, staff scientist and technical business development manager at Omega. ‘The design of the system makes it ideal for imaging of fluorescently labelled tissue as well, although few fluorescent labels are FDA-approved at this time.’
The system uses a pulsed laser beam to excite the sample in a raster pattern, while the fluorescence and reflected laser signal are directed through a series of short wavelength reflectors positioned on the ends of optical fibre tips and then into a single high gain, high bandwidth PMT. This design allows for the detection of up to 14 channels (a combination of reflected and fluorescent channels) within 2.5 microseconds per pixel. ‘We have also demonstrated the system as a detection scheme for flow cytometry,’ Locknar continued. ‘This would introduce a new method of imaging flow cytometry which would provide an alternative for the time-delay integration on a CCD detector already on the market. Our system provides a larger number of channels in a robust optical system that is relatively small, portable and resistant to vibration, given the reliance on optical fibres instead of free-space optics.’