A team of researchers at the School of Physics at the University of St Andrews has developed tiny lasers that could revolutionise the understanding and treatment of many diseases, including cancer, by enabling doctors to track individual cells throughout the body.
In Nature Communications, the team describes how its newly developed lasers – taking the form of tiny disks less than 1 micron in size – can be inserted into individual live cells, from where they can then send out signals that report on either the location of, or even the conditions within, each cell.
Currently, biologists typically use fluorescent dyes or fluorescent proteins to track the location of cells. Replacing these with the researchers’ tiny lasers would grant the ability to follow a much greater number of cells without losing track of which cell is which. This is because the light generated by each laser contains only a single wavelength, whereas the dyes generate light of multiple wavelengths in parallel, making it difficult to distinguish accurately the light from more than four or five different dyes – the colours simply become too much alike.
Instead, the researchers have now shown that it is possible to produce thousands of lasers that each generate light of a slightly different wavelength, and that these wavelengths can be distinguished with great certainty.
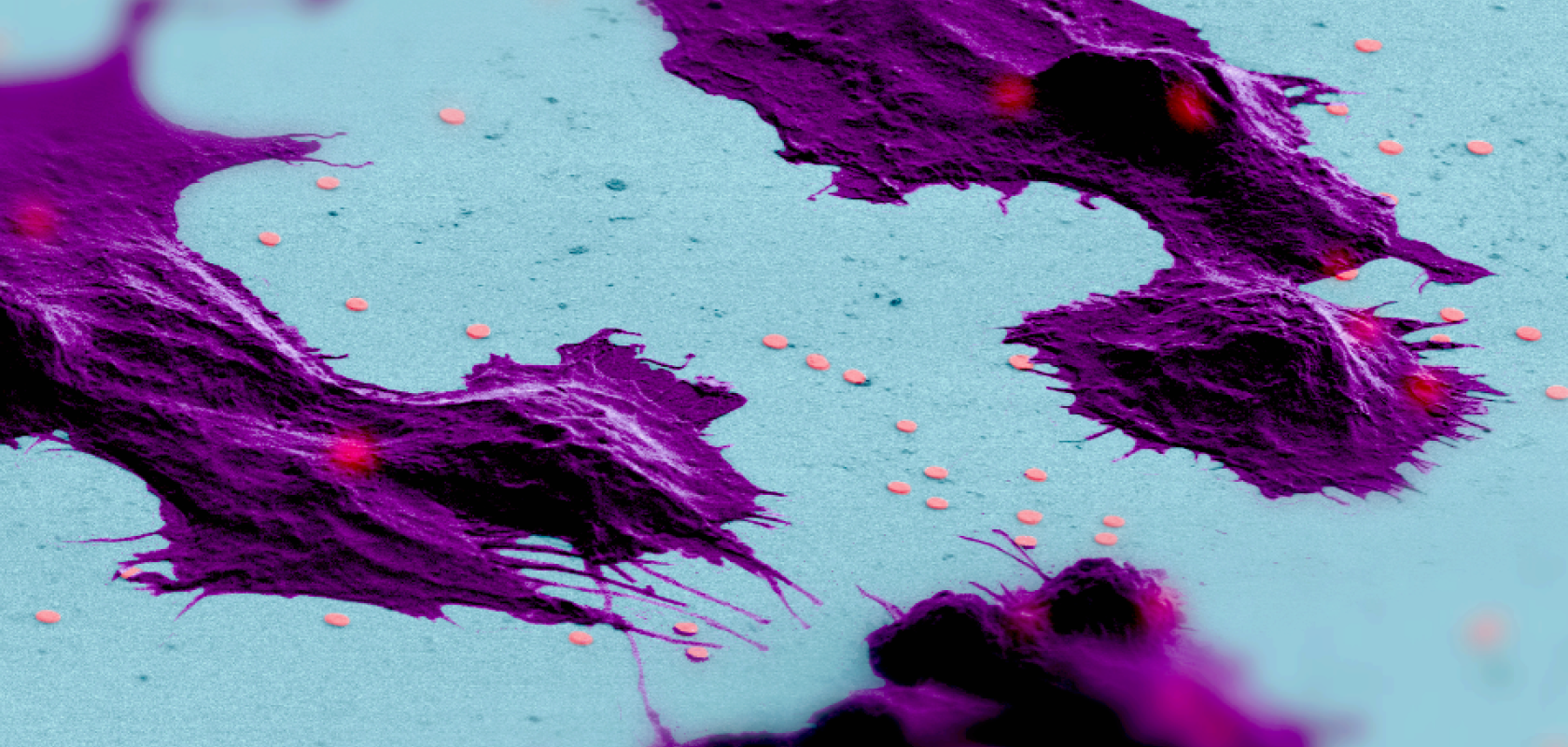
The new lasers are much smaller than the nucleus of most cells, and are made of a semiconductor quantum well material that not only enables them to provide the brightest possible laser emission, but also ensures that the colour of the emission is compatible with the requirements of the cells.
While lasers have been placed inside cells before, earlier demonstrations of the technique have occupied a volume inside the cells over one thousand times larger than that of the St Andrews team’s lasers. They also required more energy to operate, which limited their application, especially for tasks like following immune cells on their path to local sites of inflammation, or monitoring the spread of cancer cells through tissue.
Lead academic Professor Malte Gather, from the School of Physics and Astronomy, said: ‘While it is exciting to think of cyborg immune cells that fight off bacteria with an ‘on-board laser cannon’, the real value of the latest research is more likely in enabling new ways of observing cells and thus better understanding the mechanisms of disease.’
Dr Andrea Di Falco, from the School of Physics and Astronomy, who co-supervised the project, added: ‘Our work is enabled by sophisticated nanotechnology. A new nanofabrication facility here in St Andrews allows us to produce lasers that are among the smallest known to date. These internalised sensors, akin to RFID microchips, permit to follow the cells as they feed, interact with their neighbours and move through narrow obstacles, without conditioning their behaviour.’
PhD student Alasdair Fikouras and Royal Society Fellow Dr Marcel Schubert, who jointly tested the new lasers, are very excited about the prospects of the new laser platform: ‘The new lasers can help us study so many urgent questions in completely different ways than before. We can now follow individual cancer cells to understand when and how they become invasive. It’s biology on the single cell level that makes it so powerful.
Image caption: Electron microscopy image of cells (coloured in purple) that are in the process of internalizing the new nano-lasers (coloured in red). Credit: A Fikouras / U St Andrews.